Gas Pressure: Definition, Units, Measurement
– In this subject, we will discuss Gas Pressure: Definition, Units, Measurement
Key points of the Gas Pressure lesson
:(Key points of this lesson (gas pressure
Firstly, Pressure, force divided by area, provides a criterion of mechanical equilibrium for systems free to change their volume
Secondly, Pressure is measured with a barometer.
The states of gases
– The physical state of a sample of a substance, its physical condition, is defined by its physical properties.
– Two samples of a substance that have the same physical properties are in the same state.
– The state of a pure gas, for example, is specified by giving its volume, V, amount of substance (number of moles), n, pressure, p, and temperature, T.
– However, it has been established experimentally that it is sufficient to specify only three of these variables, for then the fourth variable is fixed.
– That is, it is an experimental fact that each substance is described by an equation of state, an equation that interrelates these four variables. So, The general form of an equation of state is:
p = f (T, V, n)
The general form of an equation of state
Definition of Gas pressure
– Pressure, p, is defined as force, F, divided by the area, A, to which the force is applied: p = F / A
– The greater the force acting on a given area, the greater the pressure.
– The origin of the force exerted by a gas is the incessant battering of the molecules on the walls of its container.
– The collisions are so numerous that they exert an effectively steady force, which is experienced as a steady pressure.
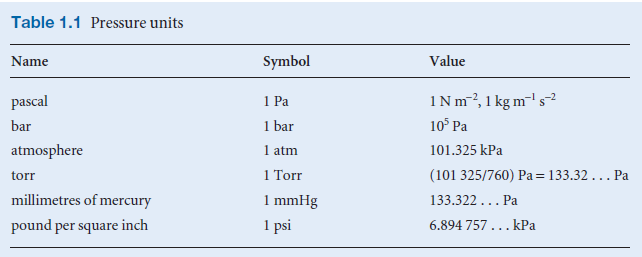
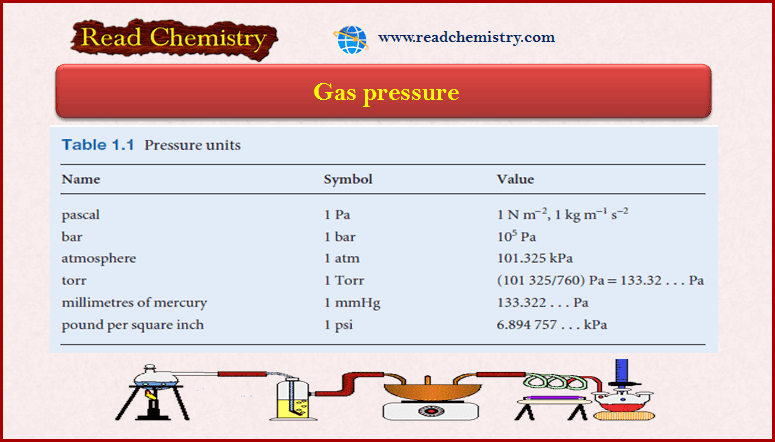
– The SI unit of pressure is the Pascal (Pa, 1 Pa = 1 N/ m2).
Gas Pressure units
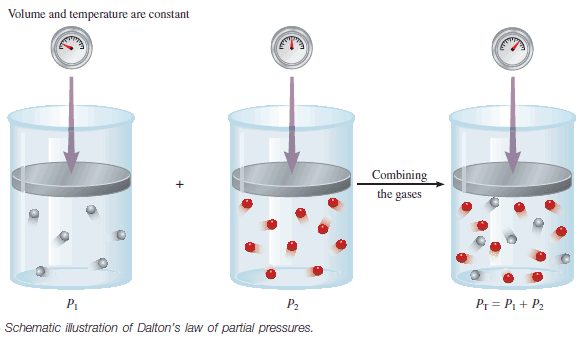
– If two gases are in separate containers that share a common movable wall, the gas that has the higher pressure will tend to compress (reduce the volume of ) the gas that has the lower pressure.
– The pressure of the high-pressure gas will fall as it expands and that of the low-pressure gas will rise as it is compressed.
– There will come a stage when the two pressures are equal and the wall has no further tendency to move.
– This condition of equality of pressure on either side of a movable wall is a state of mechanical equilibrium between the two gases.
– The pressure of a gas is therefore an indication of whether a container that contains the gas will be in mechanical equilibrium with another gas with which it shares a movable wall.
Conclusion
(1) When a region of high pressure is separated from a region of low pressure by a movable wall, the wall will be pushed into one region or the other, as in (a) and (c).
(2) However, if the two pressures are identical, the wall will not move (b). The latter condition is one of mechanical equilibrium between the two regions.
Measurement of Gas Pressure
– The pressure exerted by the atmosphere is measured with a barometer.
– The original version of the barometer (which was invented by Torricelli, a student of Galileo) was an inverted tube of mercury sealed at the upper end.
– When the column of mercury is in mechanical equilibrium with the atmosphere, the pressure at its base is equal to that exerted by the atmosphere.
– It follows that the height of the mercury column is proportional to the external pressure.
Calculating the Pressure exerted by a column of liquid
– Derive an equation for the pressure at the base of a column of liquid of mass density ρ (rho) and height h at the surface of the Earth.
– The pressure exerted by a column of liquid is commonly called the ‘hydrostatic pressure’.
Method
– F = mg. So, to calculate F we need to know the mass m of the column of liquid.
– Mass equals mass density, ρ, multiplied by its volume, V: m = ρV.
– The first step, therefore, is to calculate the volume of a cylindrical column of liquid.
Answer
– Let the column have cross-sectional area A; then its volume is Ah and its mass is m = ρAh.
– The force the column of this mass exerts at its base is:
F = mg = ρAhg
– The pressure at the base of the column is therefore:
p= F/A = ρAhg/A = ρgh
– Note that the hydrostatic pressure is independent of the shape and cross-sectional area of the column.
– The mass of the column of a given height increases as the area, but so does the area on which the force acts, so the two cancel.
– The pressure at the base of a column of liquid of length l is held at an angle θ (theta) to the vertical.
p = ρgl cosθ
Pressure gauge
– The pressure of a sample of gas inside a container is measured by using a pressure gauge.
– It is a device with electrical properties that depend on the pressure.
– The Bayard–Alpert pressure gauge is based on the ionization of the molecules present in the gas and the resulting current of ions is interpreted in terms of the pressure.
– In a capacitance manometer, the deflection of a diaphragm relative to a fixed electrode is monitored through its effect on the capacitance of the arrangement.
– Certain semiconductors also respond to pressure and are used as transducers in solid-state pressure gauges.
Reference: Physical Chemistry: The Essential Concepts /Peter Atkins, Julio de Paula. ( ninth edition) .