Nomenclature of Acids, Bases and Hydrates
– In this subject, we will discuss the Nomenclature of Acids, Bases and Hydrates
Nomenclature of Acids
– An acid can be described as a substance that yields hydrogen ions (H+) when dissolved in water. (H+ is equivalent to one proton, and is often referred to that way.)
– Formulas for acids contain one or more hydrogen atoms as well as an anionic group.
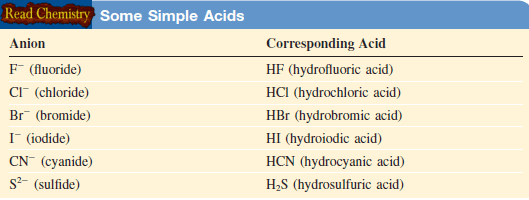
– Anions whose names end in “-ide” have associated acids with a “hydro-” prefix and an “-ic” ending, as shown in this Table:
– In some cases, two different names are assigned to the same chemical formula.
– For instance, HCl is known as both hydrogen chloride and hydrochloric acid.
– The name used for this compound depends on its physical state.
– In the gaseous or pure liquid state, HCl is a molecular compound called hydrogen chloride.
– When it is dissolved in water, the molecules break up into H+ and Cl–ions; in this condition, the substance is called hydrochloric acid.
– The figure below indicates When dissolved in water, the HCl molecule is converted to the H+ and Cl– ions.
– The H+ ion is associated with one or more water molecules and is usually represented as H3O+.

Oxoacids
– Oxoacids are acids that contain hydrogen, oxygen, and another element (the central element).
– The formulas of oxoacids are usually written with the H first, followed by the central element, and then O.
– We use the following five common acids as our references in naming oxoacids:
– Often two or more oxoacids have the same central atom but a different number of O atoms.
– Starting with our reference oxoacids, whose names all end with “-ic,”
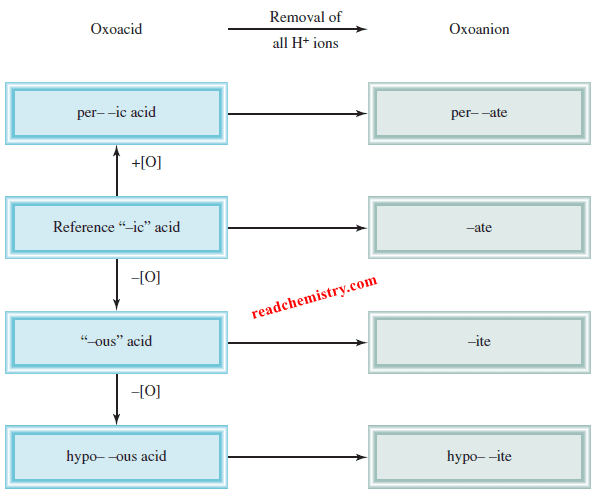
The rules for naming oxoacids
(1) Addition of one O atom to the “-ic” acid: The acid is called “per . . . -ic” acid. Thus, adding an O atom to HClO3 changes chloric acid to perchloric acid, HClO4.
(2) Removal of one O atom from the “-ic” acid: The acid is called “-ous” acid. Thus, nitric acid, HNO3, becomes nitrous acid, HNO2.
(3) Removal of two O atoms from the “-ic” acid: The acid is called “hypo . . . -ous” acid. Thus, when HBrO3 is converted to HBrO, the acid is called hypobromous acid.
The rules for naming anions of oxoacids (oxoanions)
(1) When all the H ions are removed from the “-ic” acid, the anion’s name ends with “-ate.” For example, the anion CO3 2- derived from H2CO3 is called carbonate.
(2) When all the H ions are removed from the “-ous” acid, the anion’s name ends with “-ite.” Thus, the anion ClO2– derived from HClO2 is called chlorite.
(3) The names of anions in which one or more but not all of the hydrogen ions have been removed must indicate the number of H ions present. For example, consider the anions derived from phosphoric acid:
– Note that we usually omit the prefix “mono-” when there is only one H in the anion.
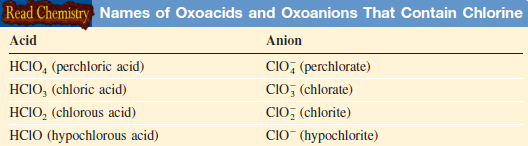
– This Table gives the names of the oxoacids and oxoanions that contain chlorine:
– This Figure summarizes the nomenclature for the oxoacids and oxoanions:
Example: Name the following oxoacid and oxoanion: (a) H3PO3 and (b) IO–4.
Strategy:
– We refer to the Figure and Table found for the conventions used in naming oxoacids and oxoanions.
Solution:
(a) We start with our reference acid, phosphoric acid (H3PO4).
– Because H3PO3 has one fewer O atom, it is called phosphorous acid.
(b) The parent acid is HIO4.
– Because the acid has one more O atom than our reference iodic acid (HIO3), it is called periodic acid.
– Therefore, the anion derived from HIO4 is called periodate.
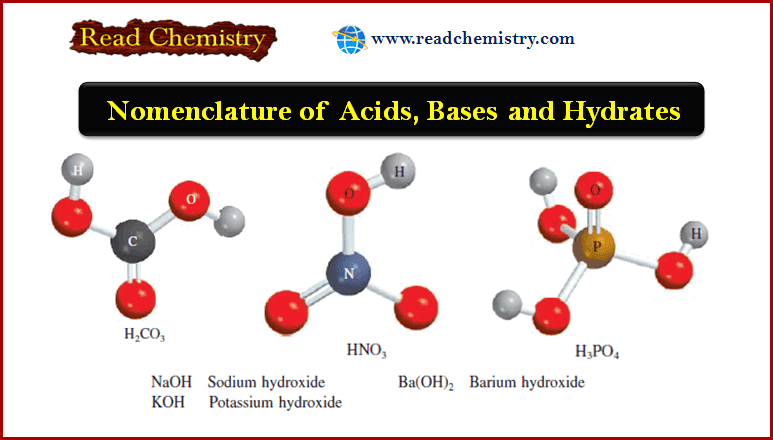
Nomenclature of Bases
– A base can be described as a substance that yields hydroxide ions (OH–) when dissolved in water.
– Some examples are:
– Ammonia (NH3), a molecular compound in the gaseous or pure liquid state, is also classified as a common base.
– At first glance, this may seem to be an exception to the definition of a base.
– Note that as long as a substance yields hydroxide ions when dissolved in water, it need not contain hydroxide ions in its structure to be considered a base.
– when ammonia dissolves in water, NH3 reacts partially with water to yield NH4+ and OH– ions. Thus, it is properly classified as a base.
Nomenclature of Hydrates
– Hydrates are compounds that have a specific number of water molecules attached to them.
– For example, in its normal state, each unit of copper(II) sulfate has five water molecules associated with it.
– The systematic name for this compound is copper(II) sulfate pentahydrate, and its formula is written as CuSO4.5H2O.
– The water molecules can be driven off by heating.
– When this occurs, the resulting compound is CuSO4, which is sometimes called anhydrous copper(II) sulfate; “anhydrous” means that the compound no longer has water molecules associated.
– Some other hydrates are:
Reference: General Chemistry: The Essential Concepts / Raymond Chang, Jason Overby. (sixth edition) .