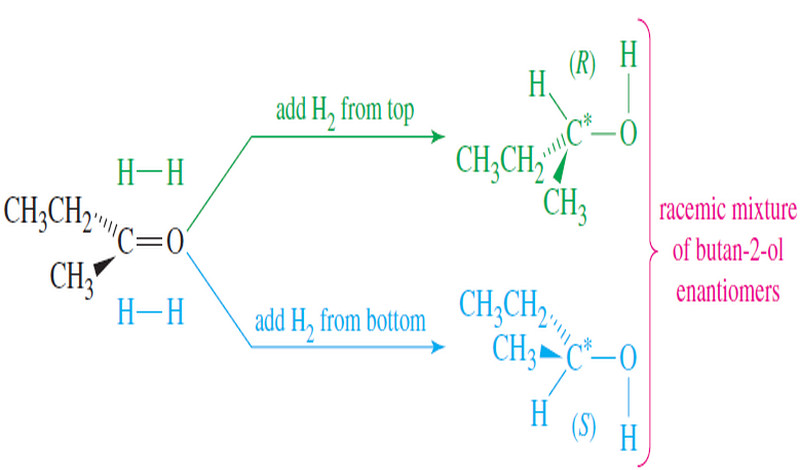
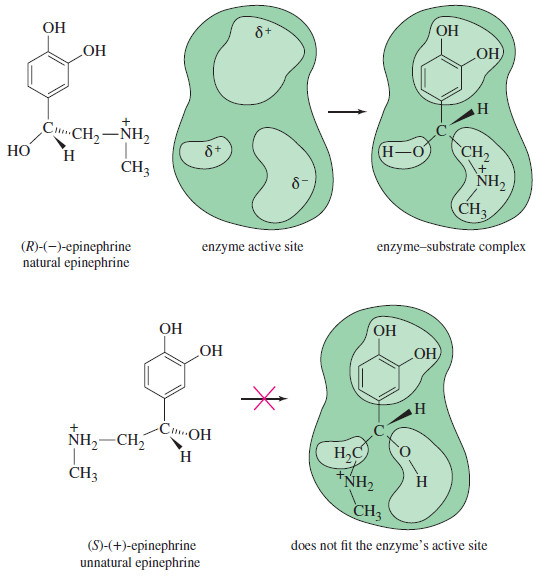
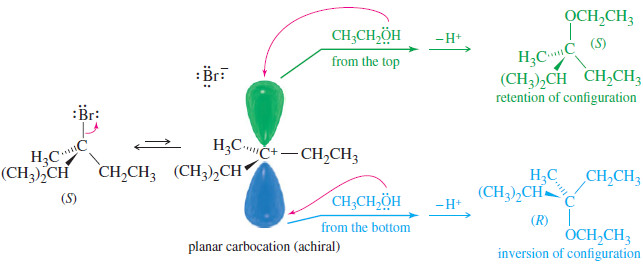
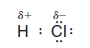Polar Covalent Bond and Dipole moment
– In this subject, we will discuss the Polar Covalent Bond and Dipole moment.
Polar Covalent Bonds
– Covalent bonds form by sharing of electrons between atoms of similar electronegativities to achieve the configuration of a noble gas.
– If we have a compound such as LiF in which the bond is between two atoms with very large electronegativity differences.
– In instances like these, a complete transfer of electrons occurs, giving the compound an ionic bond.
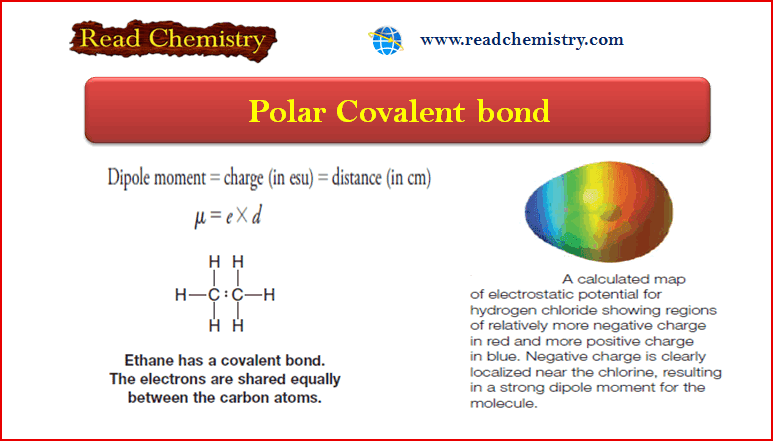
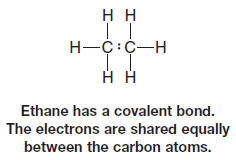
– We also described molecules in which electronegativity differences are not large, or in which they are the same, such as the carbon-carbon bond of ethane.
– Here the electrons are shared equally between the atoms.
– Until now, we have not considered the possibility that the electrons of a covalent bond might be shared unequally.
– If electronegativity differences exist between two bonded atoms, and they are not large, the electrons are not shared equally and a polar covalent bond is the result.
– Remember: one definition of electronegativity is the ability of an atom to attract electrons that it shares in a covalent bond.
Polar covalent bond in Hydrogen Chloride
– An example of such a polar covalent bond is the one in hydrogen chloride.
– The chlorine atom, with its greater electronegativity, pulls the bonding electrons closer to it.
– This makes the hydrogen atom somewhat electron-deficient and gives it a partial positive charge (δ+).
– The chlorine atom becomes somewhat electron-rich and bears a partial negative charge (δ-):
– Because the hydrogen chloride molecule has a partially positive end and a partially negative end, it is a dipole, and it has a dipole moment.
– The direction of polarity of a polar bond can be symbolized by a vector quantity +→ The crossed end of the arrow is the positive end and the arrowhead is the negative end:
– In HCl, for example, we would indicate the direction of the dipole moment in the following way:
Dipole moment
– The dipole moment is a physical property that can be measured experimentally.
– It is defined as the product of the magnitude of the charge in electrostatic units (esu) and the distance that separates them in centimeters (cm):
– The charges are typically on the order of 10-10 esu and the distances are on the order of 10-8 cm.
– Dipole moments, therefore, are typically on the order of 10-18 esu cm.
– For convenience, this unit, 1 × 10-18 esu cm, is defined as one debye and is abbreviated (D).
– The unit is named after Peter J. W. Debye, a chemist born in the Netherlands and who taught at Cornell University from 1936 to 1966. Debye won the Nobel Prize in Chemistry in 1936.
– In SI units 1 D = 3.336 × 10-30 coulomb meter (C . m).
– If necessary, the length of the arrow can be used to indicate the magnitude of the dipole moment.
– Dipole moments are very useful quantities in accounting for the physical properties of compounds.
Notes
– Polar covalent bonds strongly influence the physical properties and reactivity of molecules.
– In many cases, these polar covalent bonds are part of functional groups, which we shall study shortly.
– Functional groups are defined groups of atoms in a molecule that give rise to the function (reactivity or physical properties) of the molecule.
– Functional groups often contain atoms having different electronegativity values and unshared electron pairs.
– Atoms such as oxygen, nitrogen, and sulfur that form covalent bonds and have unshared electron pairs are called Heteroatoms.
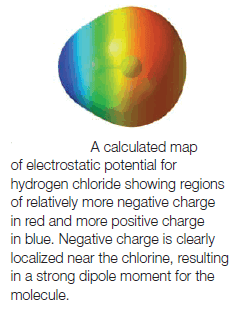
Maps of Electrostatic Potential
– One way to visualize the distribution of charge in a molecule is with a map of electrostatic potential (MEP).
(1) Regions of an electron density surface that are more negative than others in an MEP are colored red.
– These regions would attract a positively charged species (or repel a negative charge).
(2) Regions in the MEP that are less negative (or are positive) are blue.
– Blue regions are likely to attract electrons from another molecule.
(3) The spectrum of colors from red to blue indicates the trend in charge from most negative to least negative (or most positive).
– The Figure above shows a map of the electrostatic potential for the low-electron-density surface of hydrogen chloride.
– We can see that:
– Negative charge is concentrated near the chlorine atom and that positive charge is localized near the hydrogen atom, as we predict based on the difference in their electronegativity values.
– Furthermore, because this MEP is plotted at the low-electron-density surface of the molecule (the van der Waals surface) it also indicates the molecule’s overall shape.
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.