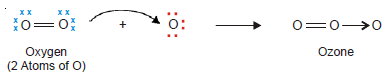Coordinate Bond: Definition, Formation, Examples
– In this subject, we will discuss the Coordinate Bond: (Definition, Formation, Examples)
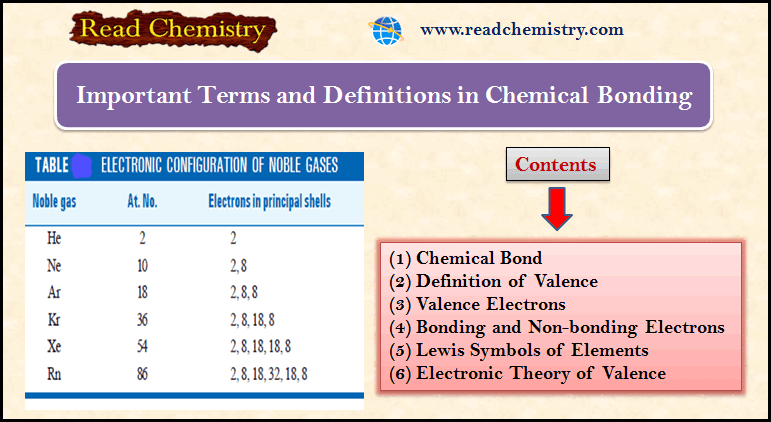
Coordinate bond
– In a normal covalent bond, each of the two bonded atoms contributes one electron to make the shared pair.
– In some cases, a covalent bond is formed when both electrons are supplied entirely by one atom.
– Such a bond is called a coordinate bond, a coordinate covalent bond or a dative bond.
– A coordinate bond is defined as a covalent bond in which both electrons of the shared pair come from one of the two atoms (or ions).
– The compounds containing a coordinate bond are called coordinate compounds.
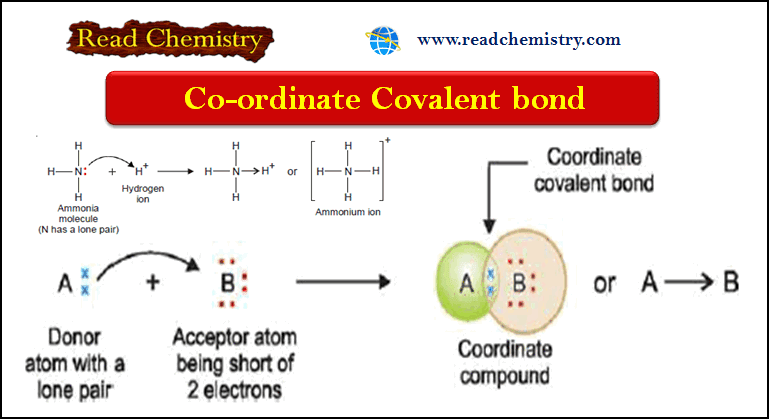
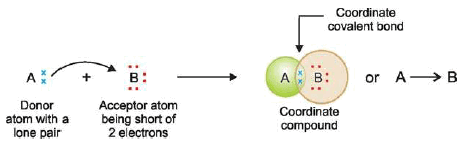
– If an atom A has an unshared pair of electrons (lone pair) and another atom B is short of two electrons than the stable number, a coordinate bond is formed.
– A donates the lone pair to B which accepts it.
– Thus both A and B achieve the stable 2 or 8 electrons, the lone pair being held in common The atom A which donates the lone pair is called the donor, while B which accepts it is the acceptor.
– The bond thus established is indicated by an arrow pointing from A to B.
– Although the arrowhead indicates the origin of the electrons, once the coordinate bond is formed it is in no way different from an ordinary covalent bond.
– The molecule or ion that contains the donor atom is called the ligand.
Some Examples of coordinate compounds or ions
– Lewis structures of some common molecules or ions containing a coordinate covalent bond are listed below:
Coordinate bond in Ammonium ion, NH4+
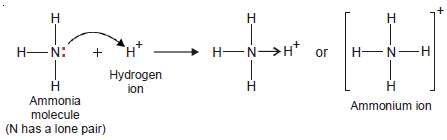
– In an ammonia molecule, the central N atom is linked to three H atoms and yet N has an unshared pair of electrons.
– The H+ion furnished by an acid has no electron to contribute and can accept a pair of electrons loaned by N atom.
– Thus, NH3donates its unshared electrons to H+ forming ammonium ion.
– All the N–H bonds in NH4+ are identical, once the coordinate bond N→H+ is established
Hydronium ion, H3O+
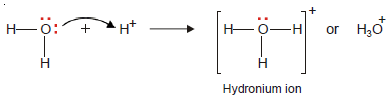
– The oxygen atom in a water molecule is attached to two H atoms by two covalent bonds.
– There are still two unshared pairs of electrons with the O atom.
– The O atom donates one of these pairs of electrons to the H+ ion and the hydronium ion is thus formed.
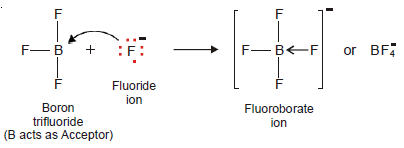
Fluoroborate ion, BF4–
– It is formed when a boron trifluoride molecule (BF3) shares a pair of electrons supplied by fluoride ion (F–).
Addition compound of NH3 with BCl3
– The N atom of ammonia molecule (NH3) has a lone pair while the B atom in boron trichloride (BCl3) is short of two electrons than a stable octet.
– An addition compound is formed as the N atom donates its lone pair to B atom of BCl3.
Coordinate bond in Nitromethane, CH3NO2
– The Lewis structure of nitromethane is shown below.
– Here the N atom has five valence electrons, three of which are used in forming a covalent bond with the C atom and two covalent bonds with the O atom.
– The N atom is still left with two unshared electrons which are donated to another O atom.
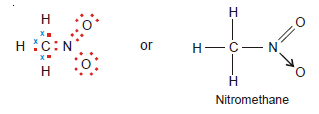
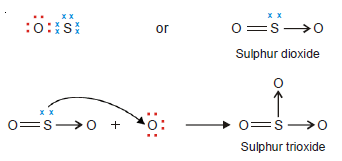
Sulphur dioxide, SO2, and Sulphur trioxide, SO3
– Sulphur achieves its octet by forming two covalent bonds with one O atom, giving SO.
– The S atom in SO has two lone pairs, one of which is shared with a second O atom to form sulphur dioxide, SO2.
– The S atom in SO2 still has one lone pair which it donates to a third O atom forming the sulphur trioxide (SO3) molecule.
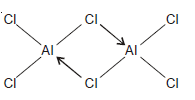
Aluminium Chloride, Al2Cl6
– The aluminium atom has three valence electrons which it shares with three Cl atoms, forming three covalent bonds.
– Thus the Al atom acquires six electrons in its outer shell.
– Now Cl atom has three lone pairs, one of which is donated to the Al atom of another molecule AlCl3.
– Thus both Al atoms achieve octet and stable Al2Cl6 results.
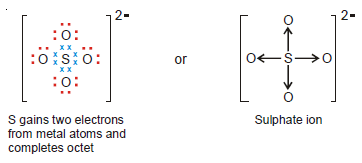
Sulphate ion, SO42-
– Sulphur has six valence electrons (2, 8, 6) and achieves the octet by gaining two electrons from metal atoms (say two Na atoms).
– The four pairs of electrons around the S atom are then donated to four oxygen atoms each of which has six electrons.
– Thus the Lewis structure for SO42- ion may be written as:
Coordinate bond in Ozone, O3
– An oxygen molecule is made of two oxygen atoms joined by two covalent bonds.
– Each O atom in O2 has two unshared pairs of electrons.
– When one pair of these is donated to a third O atom which has only six electrons, a coordinate bond is formed.
– Thus the Lewis structure of ozone may be represented as:
Carbon Monoxide, CO
– The carbon atom has four valence electrons while the oxygen atom has six.
– By forming two covalent bonds between them, the O atom achieves an octet but the C atom has only six electrons.
– Therefore O donates an unshared pair of electrons to C, and a coordinate covalent bond is established between the two atoms.
– Lewis structure of CO may be written as:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.