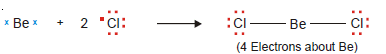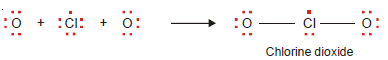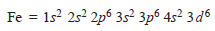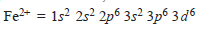Exceptions to the Octet Rule and Variable Valence
Exceptions to the octet rule
– For a time it was believed that all compounds obeyed the Octet rule or the Rule of Eight.
– However, it gradually became apparent that quite a few molecules had non-octet structures.
– Atoms in these molecules could have number of electrons in the valence shell short of the octet or in excess of the octet.
Examples of Exceptions to the octet rule
– Some important examples are:
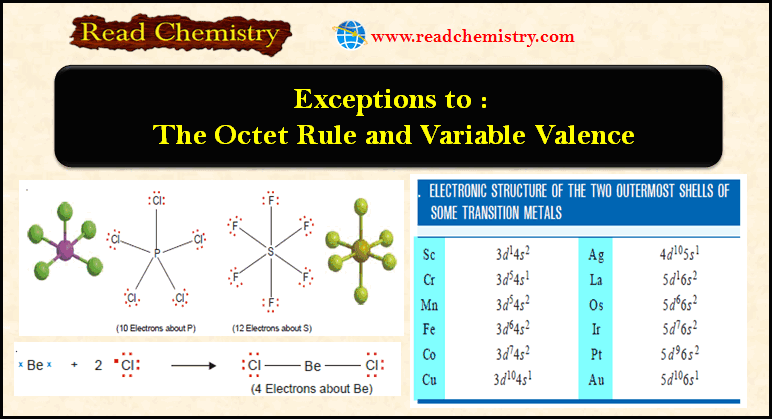
(1) Four or six electrons around the central atom
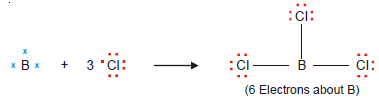
– A stable molecule of beryllium chloride, BeCl2, contains an atom with four electrons in its outer shell.
– The compound boron trifluoride, BF3, has the Lewis structure :
– The boron atom has only six electrons in its outer shell.
– Beryllium chloride and boron trifluoride are referred to as electron-deficient compounds
(2) Seven electrons around the central atom
– There are a number of relatively stable compounds in which the central atom has seven electrons in the valence shell.
– A simple example is chlorine dioxide, ClO2.
– The chlorine atom in ClO2 has seven electrons in its outer shell.
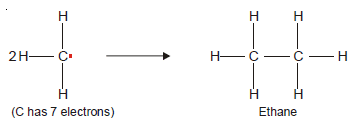
– Methyl radical (CH3) has an odd electron and is very short-lived.
– When two methyl free radicals collide, they form an ethane molecule (C2H6) to satisfy the octet of each carbon atom.
– Any species with an unpaired electron is called a free radical.
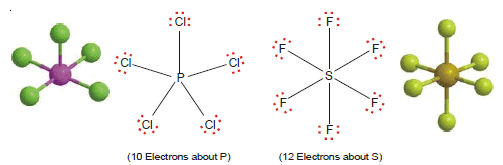
(3) Ten or more electrons around the central atom
– Non-metallic elements of the third and higher periods can react with electronegative elements to form structures in which the central atom has 10, 12, or even more electrons.
– The typical examples are PCl5 and SF6.
– The molecules with more than an octet of electrons are called super octet structures.
– In elements C, N, O, and F the octet rule is strictly obeyed because only four orbitals are available (one 2s and three 2p) for bonding.
– In the elements P and S, however, 3s, 3p, and 3d orbitals of their atoms may be involved in the covalent bonds they form.
– Whenever an atom in a molecule has more than eight electrons in its valence shell, it is said to have an expanded octet.
Variable Valence
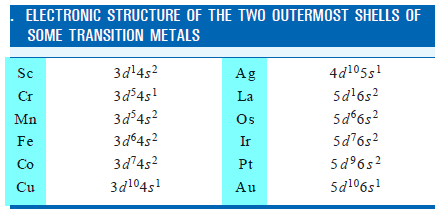
– Some elements can display two or more valences in their compounds.
– The transition metals belong to this class of elements.
– The Electronic Structure of some of these metals is given below:
– Most of the transition metals have one or two outer-shell electrons and they form monovalent or bivalent positive ions.
– But because some of the d electrons are close in energy to the outermost electrons, these can also participate in chemical bond formation.
– Thus transition metals can form ions with variable valence.
– For example, copper can form Cu1+ and Cu2+ ions and iron can form Fe2+ and Fe3+ ions.
– The complete electronic configuration of an iron atom is:
– It can form Fe2+by losing two 4s electrons,
– When iron loses two 4s electrons and one of the three 3d electrons, if forms Fe3+ion
– Copper form Cu1+ and Cu2+ ions by losing one 4s electron, and one 4s and 3d electron respectively
– It may be noted that the structures of Fe2+, Fe3+, Cu1+, Cu2+, Cr3+, etc., are not isoelectronic with any of the noble gases, and hence the d electrons being unstable are available for bond formation.
– The atoms and ions that have the same number of electrons are said to be Isoelectronic.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.