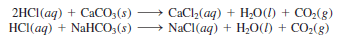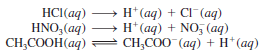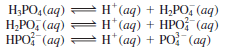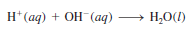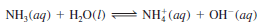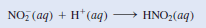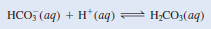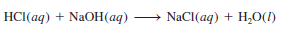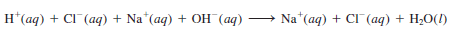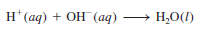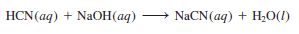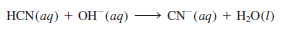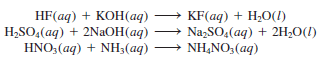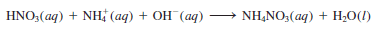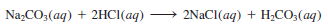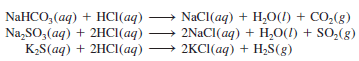Acid-Base Reactions: Definition, Examples, and Uses
– In this subject, we will discuss the Acid-Base Reactions: Definition, Examples, and Uses
– Acids and bases are as familiar as aspirin and milk of magnesia although many people do not know their chemical names—acetylsalicylic acid (aspirin) and magnesium hydroxide (milk of magnesia).
– In addition to being the basis of many medicinal and household products, acid-base chemistry is important in industrial processes and essential in sustaining biological systems.
– Before we can discuss acid-base reactions, we need to know more about acids and bases themselves.
General Properties of Acids and Bases
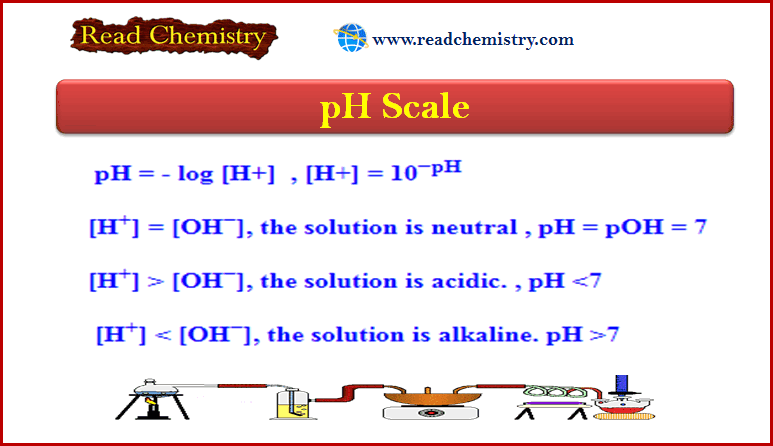
– We defined acids before as substances that ionize in water to produce H+ ions and bases as substances that ionize in water to produce OH– ions.
– These definitions were formulated in the late nineteenth century by the Swedish chemist Svante Arrhenius to classify substances whose properties in aqueous solutions were well known.
Properties of Acids
(1) Acids have a sour taste; for example, vinegar owes its sourness to acetic acid, and lemons and other citrus fruits contain citric acid.
(2) Acids cause color changes in plant dyes; for example, they change the color of litmus from blue to red.
(3) Acids react with certain metals, such as zinc, magnesium, and iron, to produce hydrogen gas.
– A typical reaction is that between hydrochloric acid and magnesium
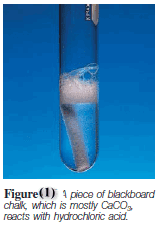
(4) Acids react with carbonates and bicarbonates, such as Na2CO3, CaCO3, and NaHCO3, to produce carbon dioxide gas ( Figure 1 ).
– For example,
(5) Aqueous acid solutions conduct electricity.
Properties of Bases
(1) Bases have a bitter taste.
(2) Bases feel slippery; for example, soaps, which contain bases, exhibit this property.
(3) Bases cause color changes in plant dyes; for example, they change the color of litmus from red to blue.
(4) Aqueous base solutions conduct electricity.
Bronsted Acids and Bases
– Arrhenius’s definitions of acids and bases are limited in that they apply only to aqueous solutions.
– Broader definitions were proposed by the Danish chemist Johannes Brønsted in 1932; a Brønsted acid is a proton donor, and a Brønsted base is a proton acceptor.
– Note that Brønsted’s definitions do not require acids and bases to be in aqueous solution.
– Hydrochloric acid is a Brønsted acid because it donates a proton in water:
– Note that the H+ ion is a hydrogen atom that has lost its electron; that is, it is just a bare proton.
– The size of a proton is about 10-15 m, compared to a diameter of 10-10 m for an average atom or ion.
– Such an exceedingly small charged particle cannot exist as a separate entity in an aqueous solution owing to its strong attraction for the negative pole (the O atom) in H2O.
– Consequently, the proton exists in the hydrated form as shown in Figure 2 .
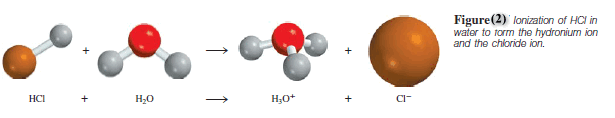
– Therefore, the ionization of hydrochloric acid should be written as:
– The hydrated proton, H3O+, is called the hydronium ion.
– This equation shows a reaction in which a Brønsted acid (HCl) donates a proton to a Brønsted base (H2O).
– Experiments show that the hydronium ion is further hydrated so that the proton may have several water molecules associated with it.
– Because the acidic properties of the proton are unaffected by the degree of hydration, in this text we will generally use H+(aq) to represent the hydrated proton.
– This notation is for convenience, but H3O+ is closer to reality. Keep in mind that both notations represent the same species in aqueous solution.
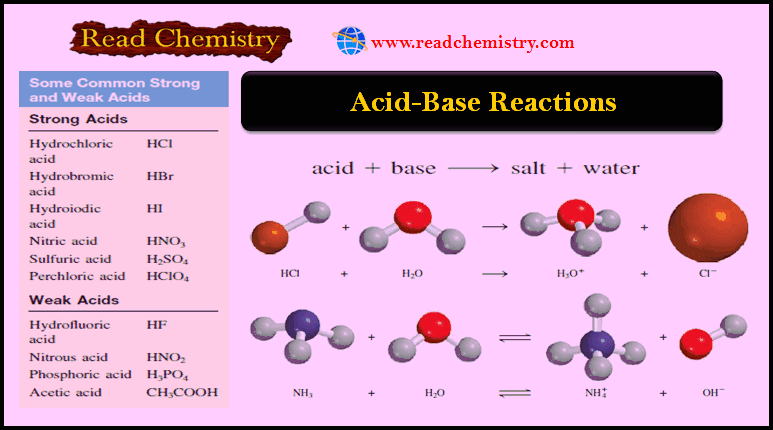
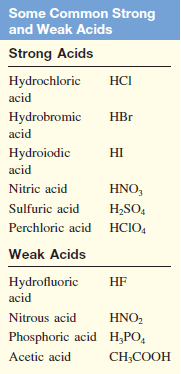
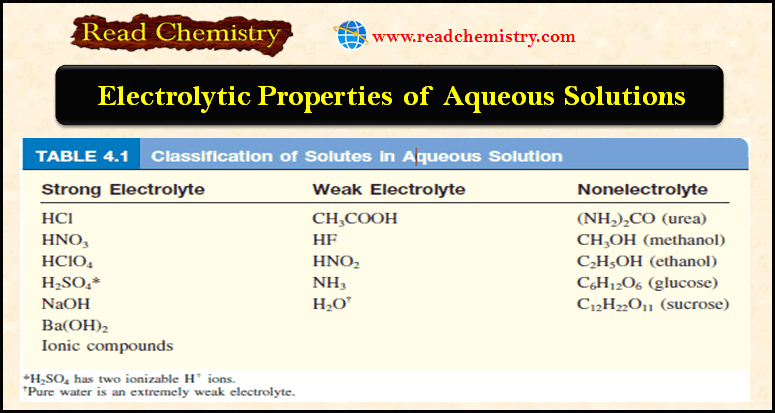
– Acids commonly used in the laboratory include hydrochloric acid (HCl), nitric acid (HNO3), acetic acid (CH3COOH), sulfuric acid (H2SO4), and phosphoric acid (H3PO4).
– The first three are monoprotic acids; that is, each unit of the acid yields one hydrogen ion upon ionization:
– As mentioned earlier, because the ionization of acetic acid is incomplete (note the double arrows), it is a weak electrolyte. For this reason, it is called a weak acid.
– On the other hand, HCl and HNO3 are strong acids because they are strong electrolytes, so they are completely ionized in solution (note the use of single arrows).
– Sulfuric acid (H2SO4) is a diprotic acid because each unit of the acid gives up two H+ ions, in two separate steps:
– H2SO4 is a strong electrolyte or strong acid (the first step of ionization is complete), but HSO4– is a weak acid or weak electrolyte, and we need a double arrow to represent its incomplete ionization.
– Triprotic acids, which yield three H+ ions, are relatively few in number.
– The best-known triprotic acid is phosphoric acid, whose ionizations are:
– All three species (H3PO4, H2PO4–, and HPO4-2) in this case are weak acids, and we use the double arrows to represent each ionization step. Anions such as H2PO4– and HPO4-2 are found in aqueous solutions of phosphates such as NaH2PO4 and Na2HPO4.
– The following Table lists several common strong and weak acids.
– sodium hydroxide (NaOH) and barium hydroxide [Ba(OH)2 ] are strong electrolytes.
– This means that they are completely ionized in solution:
– The OH–ion can accept a proton as follows:
– Thus, OH– is a Brønsted base.
– Ammonia (NH3) is classified as a Brønsted base because it can accept an H+ ion ( Figure 3):
– Ammonia is a weak electrolyte (and therefore a weak base) because only a small fraction of dissolved NH3 molecules react with water to form NH4+ and OH– ions.
– The most commonly used strong base in the laboratory is sodium hydroxide.
– It is cheap and soluble. (In fact, all of the alkali metal hydroxides are soluble.)
– The most commonly used weak base is aqueous ammonia solution, which is sometimes erroneously called ammonium hydroxide.
– There is no evidence that the species NH4OH actually exists other than the NH4+ and OH–ions in solution.
– All of the Group 2A elements form hydroxides of the type M(OH)2, where M denotes an alkaline earth metal.
– Of these hydroxides, only Ba(OH)2 is soluble.
– Magnesium and calcium hydroxides are used in medicine and industry.
– Hydroxides of other metals, such as Al(OH)3 and Zn(OH)2 are insoluble and are not used as bases.
– The following Example classifies substances as Brønsted acids or Brønsted bases.
Examples of acids and bases
Classify each of the following species in aqueous solution as a Brønsted acid or base:
(a) HBr, (b) NO2–, (c) HCO3–
Strategy:
What are the characteristics of a Brønsted acid? Does it contain at least an H atom? With the exception of ammonia, most Brønsted bases that you will encounter at this stage are anions.
Solution:
(a) We know that HCl is an acid. Because Br and Cl are both halogens (Group 7A), we expect HBr, like HCl, to ionize in water as follows:
Therefore HBr is a Brønsted acid
(b) In solution the nitrite ion can accept a proton from water to form nitrous acid:
– This property makes NO2– a Brønsted base.
(c) The bicarbonate ion is a Brønsted acid because it ionizes in solution as follows:
– It is also a Brønsted base because it can accept a proton to form carbonic acid:
Comment:
– The HCO3– species is said to be amphoteric because it possesses both acidic and basic properties.
– The double arrows show that this is a reversible reaction.
Acid-base reactions (Neutralization reactions)
– A neutralization reaction is a reaction between an acid and a base.
– Generally, aqueous acid-base reactions produce water and salt, which is an ionic compound made up of a cation other than H+ and an anion other than OH– or O2-:
Acid + Base → salt + water
– The substance we know as table salt, NaCl, is a product of the acid-base reaction
– However, because both the acid and the base are strong electrolytes, they are completely ionized in solution.
– The ionic equation is:
– Therefore, the reaction can be represented by the net ionic equation
– Both Na+ and Cl– are spectator ions.
– If we had started the preceding reaction with equal molar amounts of the acid and the base, at the end of the reaction we would have only a salt and no leftover acid or base.
– This is a characteristic of acid-base neutralization reactions.
– A reaction between a weak acid such as hydrocyanic acid (HCN) and a strong base is:
– Because HCN is a weak acid, it does not ionize appreciably in solution. Thus, the ionic equation is written as:
– and the net ionic equation is:
– Note that only Na+ is a spectator ion; OH– and CN– are not.
– The following are also examples of acid-base neutralization reactions, represented by molecular equations:
– The last equation looks different because it does not show water as a product.
– However, if we express NH3 (aq) as NH4+ (aq) and OH– (aq), as discussed earlier, then the equation becomes:
 Acid-Base Reactions Leading to Gas Formation
Acid-Base Reactions Leading to Gas Formation
– Certain salts like carbonates (containing the CO3-2 ion), bicarbonates (containing the HCO3– ion), sulfites (containing the SO3-2 ion), and sulfides (containing the S2- ion) react with acids to form gaseous products.
– For example, the molecular equation for the reaction between sodium carbonate (Na2CO3) and HCl (aq) is:
– Carbonic acid is unstable and if present in solution in sufficient concentrations decomposes as follows:
– Similar reactions involving other mentioned salts are: