Bond Dissociation Energy: Definition, Equation, Problems
– In this subject, we will discuss the Bond Dissociation Energy: Definition, Equation, Problems
Bond Dissociation Energy
– Bond breaking can be quantified using the bond dissociation energy.
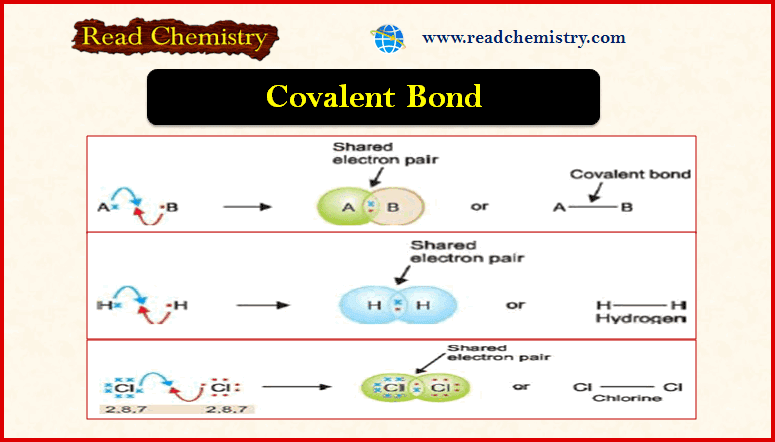
– The bond dissociation energy is the energy needed to homolytically cleave a covalent bond.
– The energy absorbed or released in any reaction, symbolized by ΔH°, is called the enthalpy change or heat of reaction.
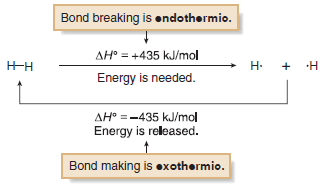
– When ΔH° is positive (+), energy is absorbed and the reaction is endothermic.
– When ΔH° is negative (–), energy is released and the reaction is exothermic.
– The superscript (°) means that values are determined under standard conditions (pure compounds in their most stable state at 25 °C and 1 atm pressure).
– A bond dissociation energy is the ΔH° for a specific kind of reaction—the homolysis of a covalent bond to form two radicals.
– Because bond breaking requires energy, bond dissociation energies are always positive numbers, and homolysis is always endothermic.
– Conversely, bond formation always releases energy, so this reaction is always exothermic.
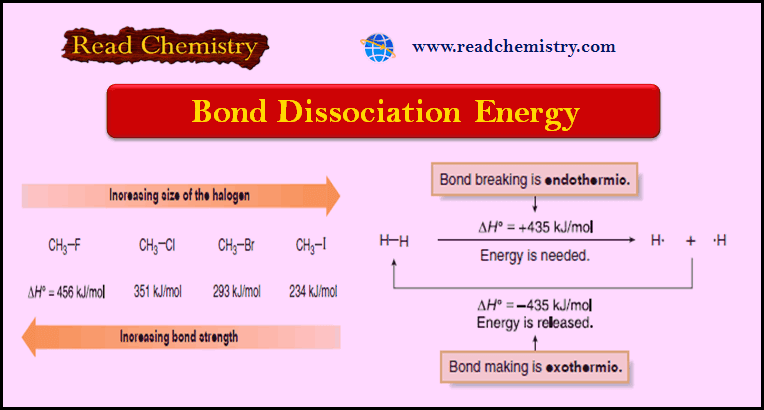
– The H– H bond requires +435 kJ/mol to cleave and releases – 435 kJ/mol when formed.
Bond dissociation energy and bond strength
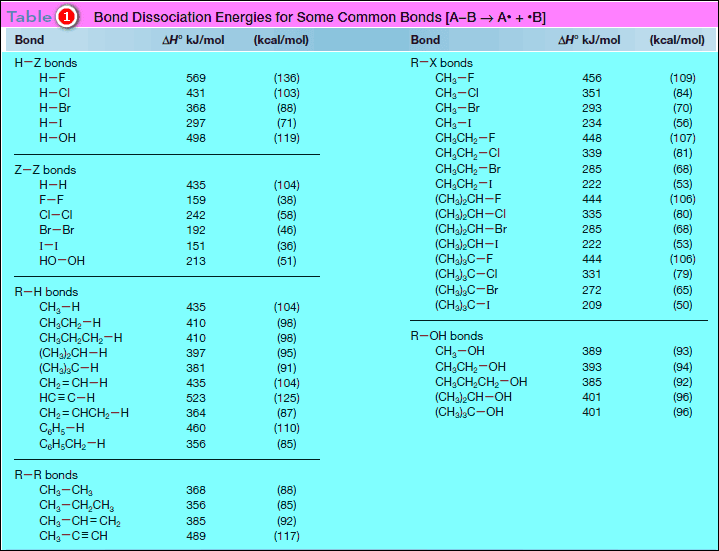
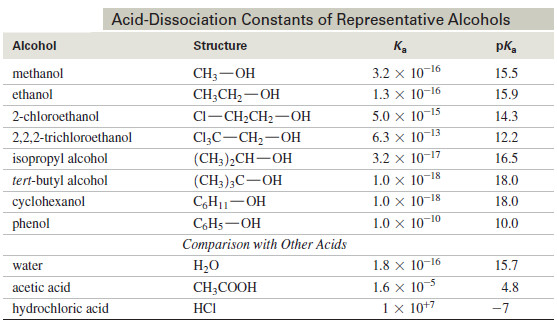
– The following Table (1) contains a representative list of bond dissociation energies for many common bonds.
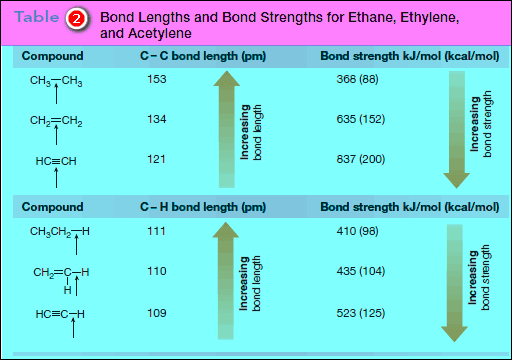
– Additional bond dissociation energies for C – C multiple bonds are given in Table (2)
– Comparing bond dissociation energies is equivalent to comparing bond strength.
– The stronger the bond, the higher its bond dissociation energy.
– For example, the H – H bond is stronger than the Cl – Cl bond because its bond dissociation energy is higher [Table: 435 kJ/mol (H2) versus 242 kJ/mol (Cl2)].
– The data in Table (1) demonstrate that bond dissociation energies decrease down a column of the periodic table as the valence electrons used in bonding are farther from the nucleus.
– Bond dissociation energies for a group of methyl–halogen bonds exemplify this trend.
Bond dissociation energy and enthalpy change
– Bond dissociation energies are also used to calculate the enthalpy change (ΔH°) in a reaction in which several bonds are broken and formed.
– ΔH° indicates the relative strength of bonds broken and formed in a reaction.
– When ΔH° is positive, more energy is needed to break bonds than is released in forming bonds.
– The bonds broken in the starting material are stronger than the bonds formed in the product.
– When ΔH° is negative, more energy is released in forming bonds than is needed to break bonds.
– The bonds formed in the product are stronger than the bonds broken in the starting material.
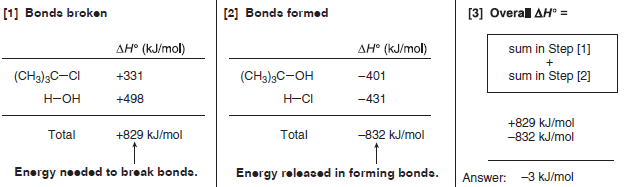
To determine the overall ΔH° for a reaction
(1) Beginning with a balanced equation, add the bond dissociation energies for all bonds broken in the starting materials.
– This (+) value represents the energy needed to break bonds.
(2) Add the bond dissociation energies for all bonds formed in the products.
– This (–) value represents the energy released in forming bonds.
(3) The overall ΔH° is the sum in Step (1) plus the sum in Step (2).
Important Trends
Solved Problems on Bond Dissociation Energy
Problem (1): Use the values in Table (1) to determine ΔH° for the following reaction.
answer:
Because ΔH° is a negative value, this reaction is exothermic and energy is released.
The bonds broken in the starting material are weaker than the bonds formed in the product.
Problem (2): Use the values in Table (1) to calculate ΔH° for each reaction. Classify each reaction as endothermic or exothermic.
answer:
– The oxidation of both isooctane and glucose, the two molecules form CO2 and H2O.
– ΔH° is negative for both oxidations, so both reactions are exothermic.
– Both isooctane and glucose release energy on oxidation because the bonds in the products are stronger than the bonds in the reactants.
Limitations of Bond Dissociation Energies
– Bond dissociation energies have two important limitations.
– They present overall energy changes only.
– They reveal nothing about the reaction mechanism or how fast a reaction proceeds.
– Moreover, bond dissociation energies are determined for reactions in the gas phase, whereas most organic reactions are carried out in a liquid solvent where solvation energy contributes to the overall enthalpy of a reaction.
– As such, bond dissociation energies are imperfect indicators of energy changes in a reaction.
– Despite these limitations, using bond dissociation energies to calculate ΔH° gives a useful approximation of the energy changes that occur when bonds are broken and formed in a reaction.
Reference: Organic chemistry / Janice Gorzynski Smith, University of Hawai’i at Manoa / (Third edition), 2011. USA









https://cse.google.co.jp/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.fr/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.es/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.es/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.it/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.hk/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.nl/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.in/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ru/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.pl/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.tw/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.id/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.cz/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.se/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.hu/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.ar/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.ar/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.fi/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.nz/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.pt/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ro/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ro/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.gr/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.ph/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.ph/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.no/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.no/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.no/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.il/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ie/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.sk/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.kr/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.kr/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.kr/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.pe/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.eg/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.lt/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.hr/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.rs/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.rs/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.si/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.by/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.lv/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ba/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ba/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.ng/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.ng/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.ng/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.cf/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.cf/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.pr/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.gt/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.uy/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.uy/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.lu/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.kw/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.kw/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.is/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.is/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.dz/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.dz/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.ke/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.tn/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.tn/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.tn/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.az/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.az/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.am/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.np/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.np/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.iq/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.iq/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.iq/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.to/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.cm/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.cm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.bd/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.bd/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.kz/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.kz/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.kz/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.ma/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.jo/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.jo/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ge/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ge/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.ge/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.lk/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.lk/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.ni/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.ni/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.md/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.mg/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.mg/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.mg/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.la/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.la/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.la/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.jm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.jm/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.jm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.cy/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.cy/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.qa/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.qa/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.sv/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.sv/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.rw/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.rw/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ps/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.ps/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.bo/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.bo/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.bs/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.bs/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.mu/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.mu/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.mu/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.mk/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.mk/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.mk/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.al/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.al/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.li/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.li/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.li/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.mn/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.mn/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.mn/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.bh/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.bh/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.kh/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.kh/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.lb/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.lb/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.tt/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.tt/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ci/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ci/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.ci/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.dj/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.dj/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.hn/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.hn/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.tz/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.tz/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.gm/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.gm/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.sn/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.sn/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.st/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.mt/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.mt/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.cat/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.bi/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.bi/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.bi/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.gg/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.gg/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.ly/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.ly/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.ly/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.na/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.na/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.me/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.cd/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.cd/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.cd/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.fm/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.fm/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.fm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.as/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.as/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.et/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.et/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.et/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.pa/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ht/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ht/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.ai/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.gi/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.gi/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.gi/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.vu/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.vu/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.vg/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.vg/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.vg/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.im/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.im/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.cf/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.pn/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.pn/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.tg/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.tg/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.vc/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.tj/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.om/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.om/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.tk/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.mz/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.mz/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.mz/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.bw/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ne/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.sm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.sm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.zw/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.zw/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.zw/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.tm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.tm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.gy/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.af/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.fj/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.fj/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.sl/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.cg/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ki/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.mw/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.mw/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.bj/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.bj/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.st/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.td/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.ao/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.ao/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.ao/url?q=https%3A%2F%2Flearnchemistry12.com