How to Predict the Outcome of acid-base reaction
– In this subject, we will discuss How to Predict the Outcome of acid-base reaction.
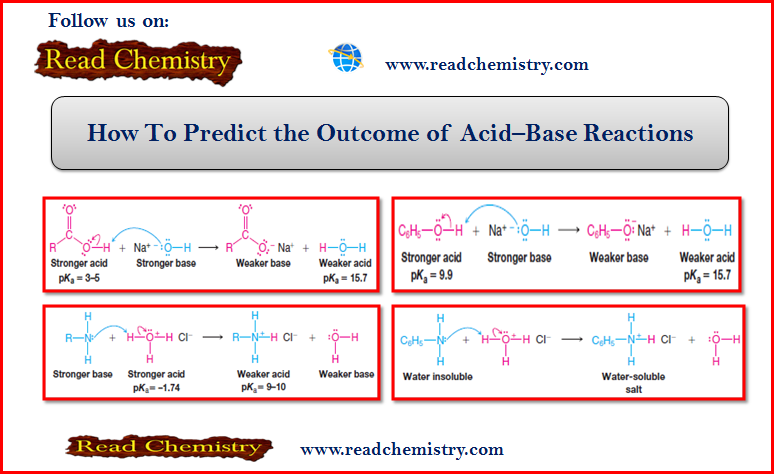
How To Predict the Outcome of acid-base reaction
– The following table gives the approximate pKa values for a range of representative compounds.
– While you probably will not be expected to memorize all of the pKa values in the table, it is a good idea to begin to learn the general order of acidity and basicity for some of the common acids and bases.
– The examples given in the table are representative of their class or functional group.
– For example, acetic acid has a pKa = 4.75, and carboxylic acids generally have pKa values near this value (in the range of pKa = 3–5).
– Ethyl alcohol is given as an example of an alcohol, and alcohols generally have pKa values near that of ethyl alcohol (in the pKa range 15–18), and so on.
– There are exceptions, of course, and we shall learn what these exceptions are as we go on.
– By learning the relative scale of acidity of common acids now, you will be able to predict whether or not an acid–base reaction will occur as written.
– The general principle to apply is this: acid–base reactions always favor the formation of the weaker acid and the weaker base.
– The reason for this is that the outcome of an acid-base reaction is determined by the position of an equilibrium.
– Acid–base reactions are said, therefore, to be under equilibrium control, and reactions under equilibrium control always favor the formation of the most stable (lowest potential energy) species.
– The weaker acid and weaker base are more stable (lower in potential energy) than the stronger acid and stronger base.
Predicting of reaction between Carboxylic acid and NaOH
– Using this principle, we can predict that a carboxylic acid (RCO2H) will react with aqueous NaOH in the following way because the reaction will lead to the formation of the weaker acid (H2O) and weaker base (RCO2–):
– Because there is a large difference in the value of the pKa of the two acids, the position of equilibrium will greatly favor the formation of the products.
– In instances like these we commonly show the reaction with a one-way arrow even though the reaction is an equilibrium.
Solved problem on the Outcome of acid-base reaction
Problem (1): Consider the mixing of an aqueous solution of phenol, C6H5OH (see Table above), and NaOH. What acid-base reaction, if any, would take place?
Strategy:
– Consider the relative acidities of the reactant (phenol) and of the acid that might be formed (water) by a proton transfer to the base (the hydroxide ion).
Answer:
– The following reaction would take place because it would lead to the formation of a weaker acid (water) from the stronger acid (phenol).
– It would also lead to the formation of a weaker base, C6H5ONa, from the stronger base, NaOH.
Problem (2): Using the Table above, explain why the acid-base reaction that takes place between NaH (as the source of CH- ions) and CH3OH is:
Answer:
– A hydride ion is a very strong base, being the conjugate base of H2 (a very weak acid, pKa = 35).
– Hydride will remove the most acidic proton from CH3OH.
– Although CH3OH is not given in the Table above, we can compare it to CH3CH2OH, a similar alcohol whose hydroxyl group pKa is 16, far more acidic than any proton attached to a carbon without a functional group (e.g., a proton of CH3CH3, which has pKa = 50).
– Because the proton attached to the oxygen is much more acidic, it is removed preferentially.
Water Solubility as the Result of Salt Formation
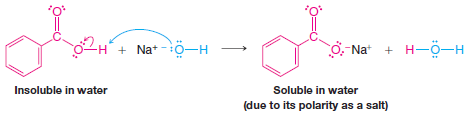
– Although acetic acid and other carboxylic acids containing fewer than five carbon atoms are soluble in water, many other carboxylic acids of higher molecular weight are not appreciably soluble in water.
– Because of their acidity, however, water-insoluble carboxylic acids dissolve in aqueous sodium hydroxide; they do so by reacting to form water-soluble sodium salts:
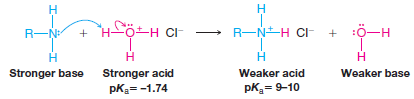
– We can also predict that an amine will react with aqueous hydrochloric acid in the following way:
– While methylamine and most amines of low molecular weight are very soluble in water, amines with higher molecular weights, such as aniline (C6H5NH2), have limited water solubility.
– However, these water-insoluble amines dissolve readily in hydrochloric acid because the acid-base reactions convert them into soluble salts:
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.