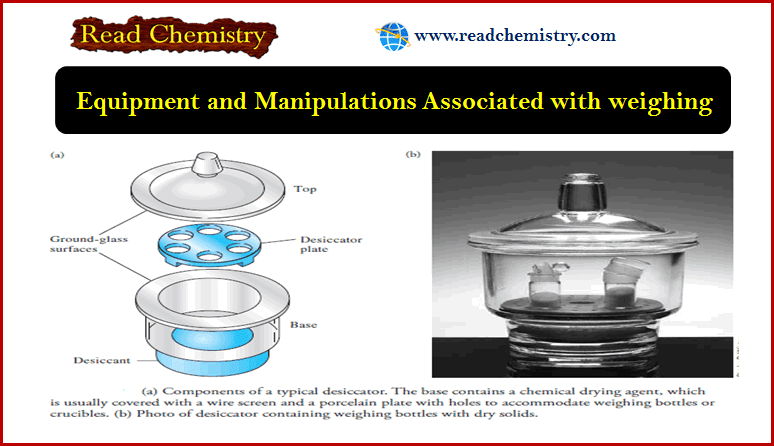
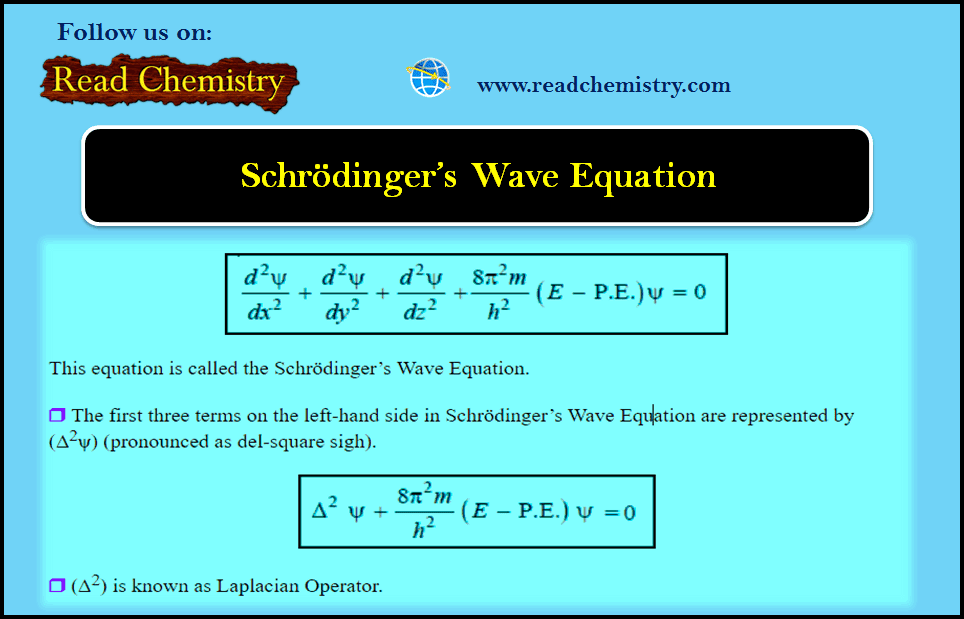
MCQ: Structure of atom – Classical Mechanics
1. In the spectrum of hydrogen atom, the series which falls in ultraviolet region is_______
(a) Lyman series
(b) Balmer series
(c) Paschen series
(d) Brackett series
Answer. (a)
2. The e/m value for the particles constituting cathode rays is the same regardless of_______
(a) the gas present in cathode rays tube
(b) the metal of which cathode was made
(c) both of these
(d) none of these
Answer. (c)
3. The charge to mass ratio (e/m) of positive particles_______
(a) varies with the nature of gas in discharge tube
(b) is independent of the gas in discharge tube
(c) is constant
(d) none of the above
Answer. (a)
4. A sub-atomic particle that has one unit mass and one unit positive charge is known as_______
(a) hydrogen atom
(b) neutron
(c) electron
(d) proton
Answer. (d)
5. Atomic number of an element is equal to the number of _______ in the nucleus of the atom.
(a) neutrons
(b) protons
(c) both the neutrons and protons
(d) electrons
Answer. (b)
6. The mass number of an atom is equal to the number of _______ in the nucleus of an atom
(a) protons
(b) neutrons
(c) electrons
(d) nucleons
Answer. (d)
7. If Z is the number of proton and A the number of nucleons, then the number of neutrons is an atom is given by_______
(a) A + Z
(b) A – Z
(c) Z – A
(d) none of these
Answer. (b)
8. The mass number and atomic number of Phosphorus atom are 31 and 15 respectively. The number of neutrons in the nucleus is_______
(a) 15
(b) 16
(c) 31
(d) 46
Answer. (b)
9. In a sodium atom (atomic number = 11 and mass number = 23), the number of neutrons is_______
(a) equal to the number of protons
(b) less than the number of protons
(c) greater than the number of protons
(d) none of these
Answer. (c)
10. Which of the following is not correct for electromagnetic waves?
(a) the wavelength is the distance between two successive crests.
(b) the frequency is the number of waves which pass a given point in one second.
(c) the velocity of a wave is the distance covered by the particular wave in one second.
(d) all electromagnetic waves have equal wavelengths.
Answer. (d)
11. Which of the following relations is not correct?
Answer. (b)
12. The unit in which wave number is measured_______
(a) hertz
(b) sec–1
(c) nanometer
(d) cm–1
Answer. (d)
13. Cathode rays are deflected by_______
(a) electric field only
(b) magnetic field only
(c) electric and magnetic field
(d) none of these
Answer. (c)
14. The Balmer series in the spectrum of hydrogen atom falls in_______
(a) ultraviolet region
(b) visible region
(c) infrared region
(d) none of these
Answer. (b)
15. The energy of a photon is given by the relation_______
Answer. (b)
16. When a beam of light of sufficiently high frequency is allowed to strike a metal surface in vacuum, electrons are ejected from the metal surface. This phenomenon is called_______
(a) Black body radiation
(b) Photoelectric effect
(c) Zeeman effect
(d) Stark effect
Answer. (b)
17. In the photoelectric effect, the kinetic energy of the photoelectrons increases linearly with the_______
(a) wavelength of the incident light
(b) frequency of the incident light
(c) velocity of the incident light
(d) none of these
Answer. (b)
18. The kinetic energy of the photoelectrons emitted from the metal surface is given by the relation_______ (vo is the threshold frequency and v is the frequency of incident light)
(a) ½ m v2 = hv – hvo
(b) ½ mv2 = hv + hvo
(c) ½ mv2= hv
(d) ½ mv2 = hvo
Answer. (a)
19. In Bohr’s model of atom, the angular momentum of an electron orbiting around the nucleus is given by the relation_______
Answer. (b)
20. The radius of first orbit in hydrogen atom according to Bohr’s Model is given by the relation_______
Answer. (a)
21. The radius of first orbit in hydrogen atom is 0.529 Å. The radius of second orbit is given by_______
(a) ½ × 0.529 Å
(b) 2 × 0.529 Å
(c) 4 × 0.529 Å
(d) 8 × 0.529 Å
Answer. (c)
22. The energy of an electron in the first orbit in hydrogen atom is –313.6/n2 kcal mol–1. The energy of the electron in 3rd orbit is given by the relation_______
Answer. (c)
23. Lyman series is obtained when the electrons from higher energy levels return to_______
(a) 1st orbit
(b) 2nd orbit
(c) 3rd orbit
(d) 4th orbit
Answer. (a)
24. A line in Pfund series is obtained when an electron from higher energy levels returns to_______
(a) 1st orbit (b) 3rd orbit
(c) 5th orbit (d) 6th orbit
Answer. (c)
25. The energy of an electron in Bohr’s atom _______ as we move away from the nucleus
(a) remains the same
(b) decreases
(c) increases
(d) sometimes increases, sometimes decreases
Answer. (c)
26. When an electron drops from a higher energy level to a lower energy level, then_______
(a) the energy is absorbed
(b) the energy is released
(c) the nuclear charge increases
(d) the nuclear charge decreases
Answer. (b)
27. The spectrum of hydrogen atom is similar to that of_______
(a) H+ ion
(b) He+ ion
(c) Li+ ion
(d) Na+ ion
Answer. (b)
28. If r is the radius of first orbit, the radius of nth orbit of hydrogen atom will be_______
(a) n2 r
(b) n r
(c) n/r
(d) r/n
Answer. (a)
29. The ratio of radii of second and first orbit of hydrogen atom according to Bohr’s model is_______
(a) 2:1
(b) 1:2
(c) 4:1
(d) 1:4
Answer. (c)
30. The spectrum of helium is expected to be similar to that of_______
(a) H-atom
(b) Li atom
(c) Li+ ion
(d) Na+ ion
Answer. (c)
31. Electromagnetic radiations with minimum wavelength is_______
(a) ultraviolet
(b) X-rays
(c) infrared
(d) radiowaves
Answer. (b)
32. Which of the following statements is false?
(a) electrons travel around the nucleus in specific permitted circular orbits
(b) an electron does not lose energy as long as it moves in its specified orbits
(c) an electron can jump from one energy level to another by absorbing or losing energy
(d) the angular momentum of an electron is not quantised
Answer. (d)
33. The idea of stationary orbits was first given by_______
(a) Rutherford
(b) JJ Thomson
(c) Niels Bohr
(d) Max Planck
Answer. (c)
34. The maximum number of electrons that can be accommodated in an orbit is_______
(a) 2n
(b) n2
(c) 2n2
(d) 2n + 1
Answer. (c)
35. The maximum number of electrons is the outermost orbit is_______
(a) 2
(b) 8
(c) 18
(d) 32
Answer. (b)
36. When the source emitting lines is placed in a strong magnetic field the spectral lines are split into its components. This effect is called_______
(a) Compton effect
(b) Zeeman effect
(c) Rydberg effect
(d) Photoelectric effect
Answer. (b)
37. The number of electrons in the outermost shell of Potassium (at. no. 19) is_______
(a) 1
(b) 2
(c) 8
(d) 9
Answer. (a)
38. An atom of silicon with atomic number 14 has the following number of electrons in the outermost shell_______
(a) 1
(b) 2
(c) 4
(d) 8
Answer. (c)
39. Inert gases possess the most stable electronic configuration as they contain_______
(a) fully filled outermost shell
(b) half filled outermost shell
(c) two electrons in the outermost shell
(d) eight electrons in the outermost shell
Answer. (d)
40. The effect of electric field on the spectra of atoms is called_______
(a) Compton effect
(b) Photoelectric effect
(c) Stark effect
(d) Zeeman effect
Answer. (c)
41. Which one of the following species has the same number of electrons as an atom of Neon?
(a) O2–
(b) Na
(c) Mg
(d) K+
Answer. (a)
42. The energy of an electron in the first Bohr orbit for hydrogen is_______
(a) 13.6 eV
(b) –13.6 eV
(c) 1.36 eV
(d) –1.36 eV
Answer. (b)
43. The energy of hydrogen atom in its ground state is –13.6 eV. The energy of the level corresponding to n = 3 is_______
(a) – 4.53 eV
(b) – 2.265 eV
(c) – 1.51 eV
(d) none of these
Answer. (c)
44. En = –1311.8 kJ mol–1. If the value of E is –52.44 kJ mol–1, to which value (n) corresponds?
(a) 2
(b) 3
(c) 4
(d) 5
Answer. (d)
45. The spectral line lies in the Lyman series. It corresponds to the radiation emitted by an electron jumping from higher energy sates to_______
(a) first energy state
(b) second energy state
(c) third energy state
(d) fifth energy state
Answer. (a)
46. The ground state of an atom corresponds to a state of_______
(a) maximum energy
(b) minimum energy
(c) zero energy
(d) negative energy
Answer. (b)
47. Balmer series in the spectrum of hydrogen atom lies in_______
(a) ultraviolet region
(b) visible region
(c) infrared region
(d) none of these
Answer. (b)
48. The spectrum of H-atom is expected to be similar to that of_______
(a) Li+
(b) Na+
(c) He+
(d) K+
Answer. (c)
49. An atom of Calcium (at. no. 20) contains _______ electrons in the third energy level.
(a) 2
(b) 8
(c) 10
(d) 18
Answer. (b)
50. Out of the following pairs of elements which has the same number of electrons in the outer most energy level?
(a) helium and lithium
(b) boron and carbon
(c) carbon and nitrogen
(d) lithium and hydrogen
Answer. (d)
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.