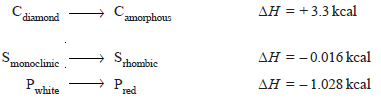Energy Changes During Transitions or Phase Changes
– In this subject, we will discuss Energy Changes During Transitions or Phase Changes.
Energy Changes During Transitions or Phase Changes
– The three states of matter – solid, liquid and gas differ from one another in the arrangement of their constituent particles.
– The magnitudes of intermolecular forces acting between the particles in these states are also different.
– It is a common observation that when a solid is converted into the liquid state, energy is to be supplied.
– This energy is spent in breaking the intermolecular forces in the solid which are of high magnitude.
– Whenever there is a change in the state of matter (solid → liquid or liquid → gas), the process is called phase change or transition.
– It is also accompanied by the change in enthalpy or heat content of the system.
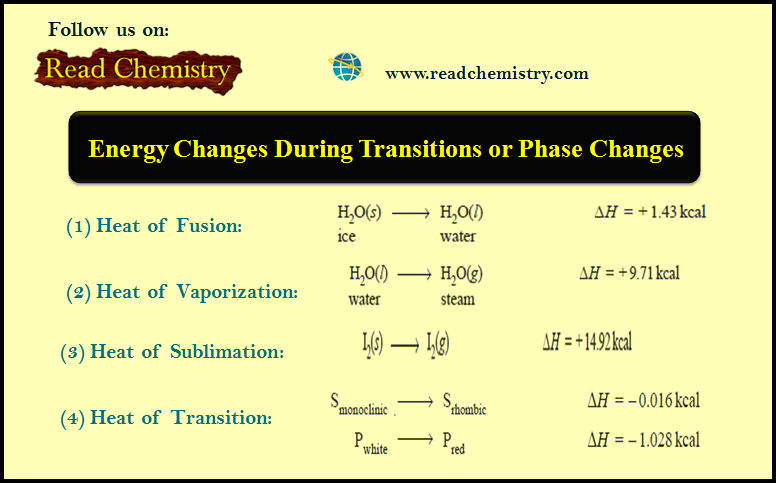
(1) Heat of Fusion
– Heat of Fusion is defined as the heat change (or enthalpy change) when one mole of a solid substance is converted into the liquid state at its melting point.
– As an example, we can take the melting of one mole of ice at its melting point, 0ºC or 273 K.
– The process can be represented as:
and is accompanied by the absorption of 1.43 kcal of heat.
– From the values of fusion of various substances we can compare their magnitudes of intermolecular forces.
– The greater the heat of fusion of a substance higher the magnitude of intermolecular forces.
(2) Heat of Vaporization
– The heat of vaporization is defined as the heat change (or enthalpy change) when one mole of liquid is converted into vapour or gaseous state at its boiling point.
– For example, when one mole of water is converted into steam at 100ºC or 373 K, the heat absorbed is 9.71 kcal which is the heat of vaporization of water.
– The change can be represented as:
– The heats of vaporization of ethyl alcohol (C2H5OH) and benzene (C6H6) are 7.29 kcal mol-1 and 7.36 kcal mol-1 respectively.
– The values of heats of vaporization can also be used for the comparison of the magnitude of intermolecular forces of attraction in liquids.
(3) Heat of Sublimation
– Sublimation is a process when a solid changes directly into gaseous state without changing into liquid state.
– It occurs at a temperature below the melting point of the solid.
– Heat of sublimation is defined as the heat change (or enthalpy change) when one mole of a solid is directly converted into the gaseous state at a temperature below its melting point.
– For example, the heat of sublimation of iodine is 14.92 kcal mol–1.
– It can be represented as:
(4) Heat of Transition
– The heat of transition is defined as the change in enthalpy which occurs when one mole of an element changes from one allotropic form to another.
– For example, the transition of diamond into amorphous carbon may be represented as:
– where – 0.016 kcal and – 1.028 kcal are heats of the transition of monoclinic sulphur to rhombic sulphur and white phosphorus to red phosphorus respectively.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.