Determination of osmotic pressure
Determination of osmotic pressure
– The osmotic pressure of a given solution can be determined experimentally by the methods detailed below.
– The apparatus used for the purpose is often referred to as osmometer.
(1) Pfeffer’s Method
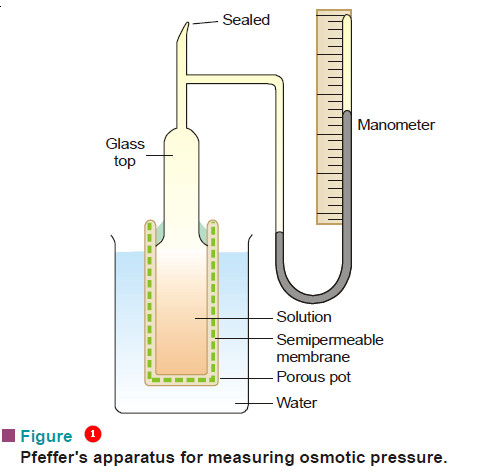
– The apparatus used by Pfeffer (1877) for determination of osmotic pressure is shown in Fig.1.
– It consists of a porous pot with copper ferrocyanide membrane deposited in its walls and having a glass top cemented to it.
– The glass top has an open tube and a closed mercury manometer in the side.
– The apparatus is filled with a solution under examination through the tube which is then sealed off.
– The pot is now placed in pure water maintained at constant temperature. The water passes across the membrane into the solution and develops a pressure on the manometer.
– The highest pressure registered by the manometer gives the osmotic pressure of the solution.
– The Pfeffer’s method suffers from two disadvantages :
(a) It is slow and it takes a few days before the highest pressure is reached.
(b) It cannot be used for measuring high osmotic pressures as the ferrocyanide membrane being weak ruptures.
Morse and Frazer (1910) used essentially the same method but deposited the copper ferrocyanide membrane electrolytically.
– The membrane so produced being more stout, they were able to measure osmotic pressures up to about 300 atmospheres.
(2) Berkeley and Hartley’s Method
– Berkeley and Hartley (1904-1909) employed the technique of applying external pressure on the solution just enough to prevent osmosis.
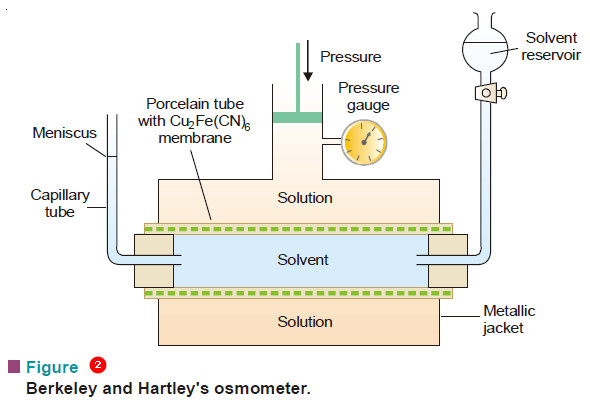
– The osmometer used by them is illustrated in Fig.2.
– A porcelain tube with copper ferrocyanide membrane deposited in its walls is enclosed in a metallic jacket.
– The tube is fitted with a reservoir of pure solvent (water) at one end and a capillary tube at the other.
– Mechanical pressure can be applied on the solution with a piston connected to a pressure gauge.
Procedure:
– The inner porcelain tube is filled with pure solvent and the jacket with the solution whose osmotic pressure is to be determined.
– The level of the solvent meniscus in the capillary tube will tend to move down as solvent flows into the solution across the membrane.
– Pressure is then applied through the piston so that the meniscus becomes stationary.
– It indicates that osmosis has been stopped and now the pressure recorded by the pressure gauge gives the osmotic pressure of the solution.
This method is superior to the older method of pfeffer in as much as :
(a) It is quick and accurate.
(b) It can be used for determining high osmotic pressures. The osmotic pressure being balanced by the external pressure, there is no strain left on the membrane and the danger of its bursting is eliminated.
(3) A Modern Osmometer
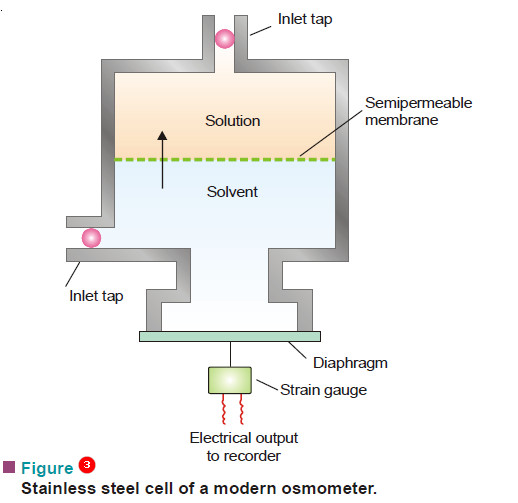
– A modern apparatus for the determination of osmotic pressure is shown in Fig.3.
– It consists of a stainless steel cell with a rigid fixed semipermeable membrane.
– The membrane divides the cell into solute and solvent compartments.
– A flexible diaphragm is fixed in the solvent compartment When the solution and the solvent compartments are filled and the taps closed, osmosis occurs.
– The solvent flows into the solution across the semipermeable membrane. This reduces the pressure in the solvent compartment, causing the diaphragm to distort.
– Eventually the pressure becomes low enough to stop the occurrence of osmosis.
– The degree of diaphragm distortion is related to the osmotic pressure of the solution.
– The diaphragm distortion is measured by a device called strain gauge. The strain gauge provides an electric current that is proportional to the extent of distortion.
– The gauge is calibrated to give osmotic pressure directly.
Isotonic solutions
– When two solutions are separated by a semipermeable membrane and there is no flow of water across the membrane, the solutions are said to be Isotonic.
– If the membrane is completely semipermeable, isotonic solutions are also iso-osmotic i.e., have the same osmotic pressure since osmotic pressure depends on the number of molecules.
– The isotonic solutions have equimolar concentrations.
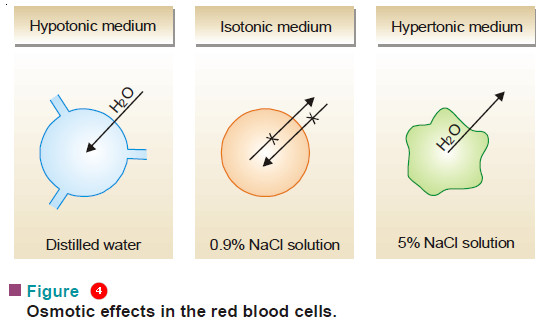
– Of the two solutions separated by a semipermeable membrane if one is of lower osmotic pressure, it is said to be hypotonic relative to the second solution.
– If it has a higher osmotic pressure than the second solution, it is said to be hypertonic relative to the second solution.
– Thus when red blood cells are placed in distilled water (hypotonic medium), water flows into the cells and they swell or burst. If, on the other hand, they are placed in 5% NaCl solution (hypertonic medium), water comes out of the cells and they shrink.
– A 0.16 M sodium chloride solution (0.95%) is isotonic with blood cells and they neither swell nor shrink as no osmosis takes place.
– However, since the membrane of the cells is not completely semipermeable and is leaky, the outside the cell and the inner fluid, although isotonic, is not necessarily iso osmotic.