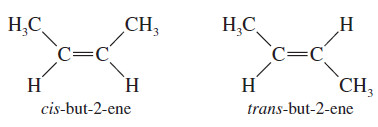What is Diastereomers?
Diastereomers
– We have defined stereoisomers as isomers whose atoms are bonded together in the same order but differ in how the atoms are directed in space.
– We have also considered enantiomers (mirror-image isomers) in detail.
– All other stereoisomers are classified as diastereomers, which are defined as stereoisomers that are not mirror images.
– Most diastereomers are either geometric isomers or compounds containing two or more chirality centers.
Cis-trans Isomerism on Double Bonds
– We have already seen one class of diastereomers, the cis-trans isomers, or geometric isomers.
– For example, there are two isomers of but-2-ene:
– These stereoisomers are not mirror images of each other, so they are not enantiomers. They are diastereomers.
Cis-trans Isomerism on Rings
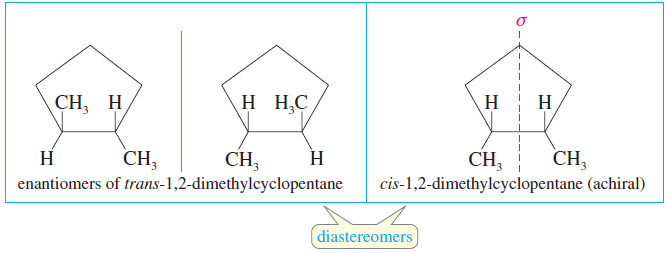
– Cis-trans isomerism is also possible when there is a ring present.
– Cis- and trans-1,2-dimethylcyclopentane are geometric isomers, and they are also diastereomers.
– The trans diastereomer has an enantiomer, but the cis diastereomer has an internal mirror plane of symmetry, so it is achiral.
Diastereomers of Molecules with Two or More Chirality Centers
– Apart from geometric isomers, most other compounds that show diastereomerism have two or more chirality centers, usually asymmetric carbon atoms.
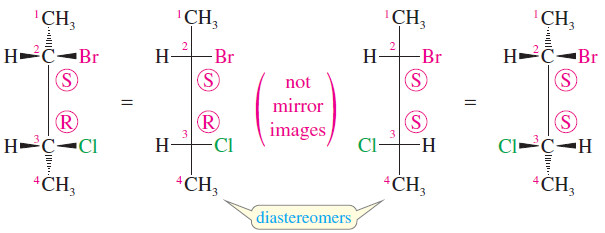
– For example, 2-bromo-3-chlorobutane has two asymmetric carbon atoms, and it exists in two diastereomeric forms (shown next).
– Make molecular models of these two stereoisomers.
– These two structures are not the same; they are stereoisomers because they differ in the orientation of their atoms in space.
– They are not enantiomers, however, because they are not mirror images of each other: C2 has the (S) configuration in both structures, while C3 is (R) in the structure on the left and (S) in the structure on the right.
– The C3 carbon atoms are mirror images of each other, but the C2 carbon atoms are not.
– If these two compounds were mirror images of each other, both asymmetric carbons would have to be mirror images of each other.
– Since these compounds are stereoisomers but not enantiomers, they must be diastereomers.
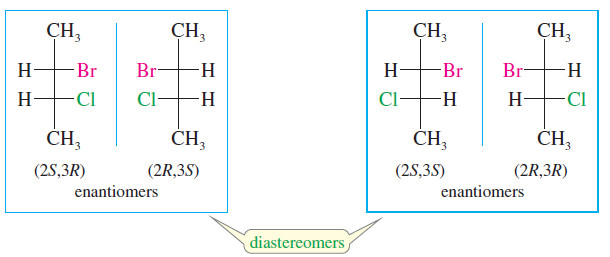
– In fact, both of these diastereomers are chiral and each has an enantiomer.
– Thus, there is a total of four stereoisomeric 2-bromo-3 chlorobutanes: two pairs of enantiomers. Either member of one pair of enantiomers is a diastereomer of either member of the other pair.
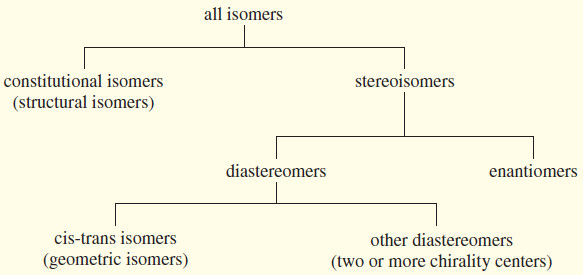
– We have now seen all the types of isomers we need to study, and we can diagram their relationships and summarize their definitions
SUMMARY: Types of Isomers
– Isomers are different compounds with the same molecular formula.
– Constitutional isomers are isomers that differ in the order in which atoms are bonded together. Constitutional isomers are sometimes called structural isomers because they have different connections among their atoms.
– Stereoisomers are isomers that differ only in the orientation of the atoms in space.
– Enantiomers are mirror-image isomers.
– Diastereomers are stereoisomers that are not mirror images of each other.
– Cis-trans isomers (geometric isomers) are diastereomers that differ in their cis-trans arrangement on a ring or double bond.