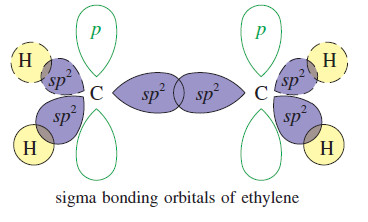
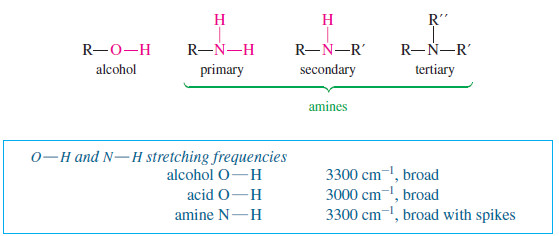
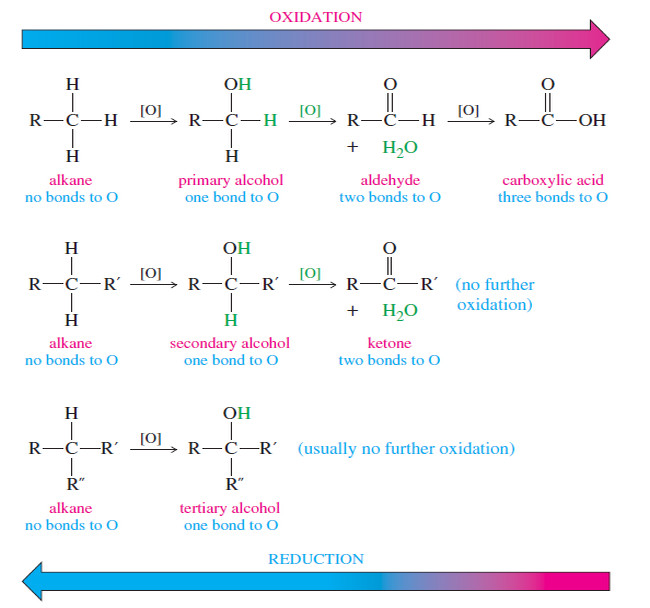
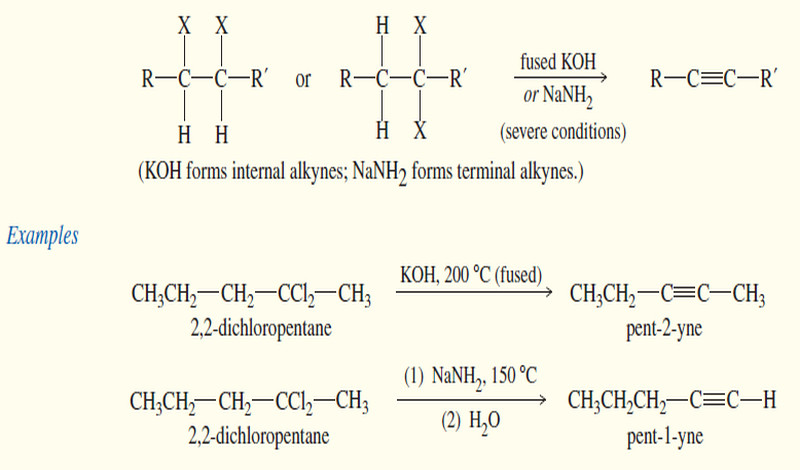
Zaitsev’s Rule : Positional Orientation of Elimination
Positional Orientation of Elimination: Zaitsev’s Rule
– In this subject , Positional Orientation of Elimination: Zaitsev’s Rule will be discussed
– Many compounds can eliminate in more than one way, to give mixtures of alkenes.
– In many cases, we can predict which elimination product will predominate.
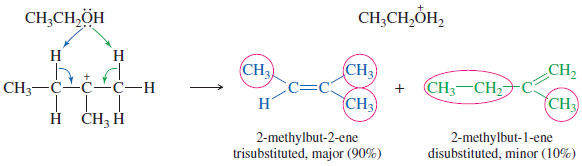
– In the example shown, the carbocation can lose a proton on either of two adjacent carbon atoms.
– The first product has a trisubstituted double bond, with three substituents (circled) on the doubly bonded carbons. It has the general formula R2C = CHR
– The second prouduct has a disubstituted double bond, with general formula R2C = CH2 (or R–CH = CH-R).
– In most E1 and E2 eliminations where there are two or more possible elimination products, the product with the most substituted double bond will predominate.
– This general principle is called Zaitsev’s rule, and reactions that give the most substituted alkene are said to follow Zaitsev orientation.
Zaitsev’s rule
Zaitsev’s rule: In elimination reactions, the most substituted alkene usually predominates.
– This order of preference is the same as the order of stability of alkenes.
– We consider the stability of alkenes in more detail in this subject (stability of alkenes) , but for now, it is enough just to know that more substituted alkenes are more stable.
– Zaitsev is transliterated from the Russian, and may also be spelled Saytzeff.
Solved problem on Zaitsev’s rule
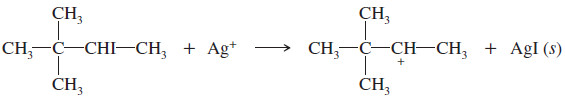
When 3-iodo-2,2-dimethylbutane is treated with silver nitrate in ethanol, three elimination products are formed. Give their structures, and predict which ones are formed in larger amounts.
SOLUTION
– Silver nitrate reacts with the alkyl iodide to give solid silver iodide and a cation.
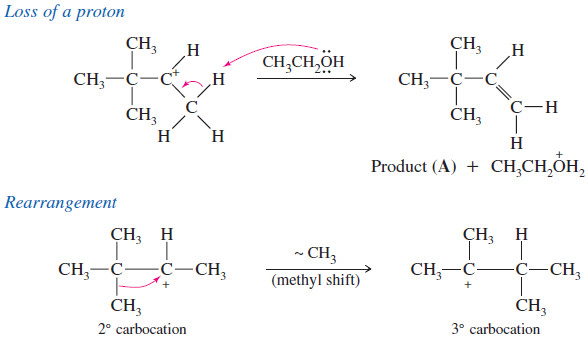
– This secondary carbocation can lose a proton to give an unrearranged alkene (A), or it can rearrange to a more stable tertiary cation
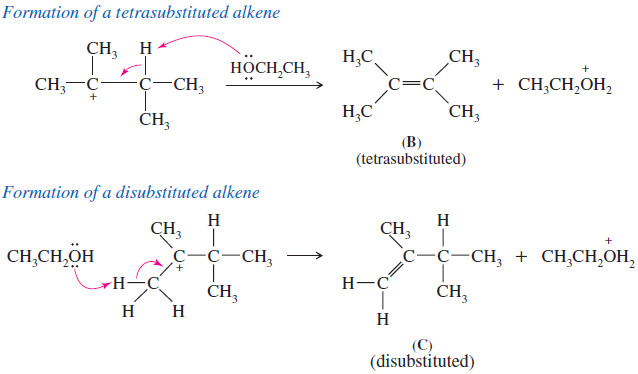
The tertiary cation can lose a proton in either of two positions. One of the products (B) is a tetrasubstituted alkene, and the other (C) is disubstituted
– Product B predominates over product C because the double bond in B is more substituted.
– Whether product A is a major product will depend on the specific reaction conditions and whether proton loss or rearrangement occurs faster