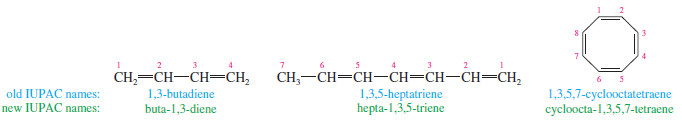Nomenclature of Alkenes
Nomenclature of Alkenes
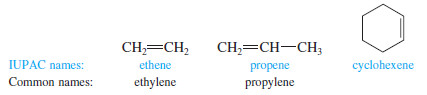
– Simple alkenes are named much like alkanes, using the root name of the longest chain containing the double bond. The ending is changed from -ane to -ene.
– For example, (ethane) becomes “ethene,” (propane) becomes “propene,” and “cyclohexane” becomes “cyclohexene”.
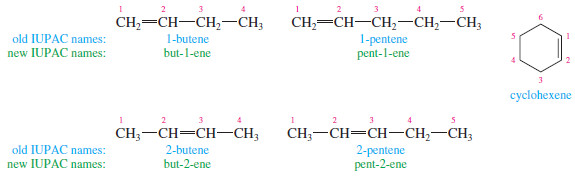
– When the chain contains more than three carbon atoms, a number is used to give the location of the double bond.
– The chain is numbered starting from the end closest to the double bond, and the double bond is given the lower number of its two double-bonded carbon atoms.
– Cycloalkenes are assumed to have the double bond in the number 1 position.
– In 1993, the IUPAC recommended a logical change in the positions of the numbers used in names.
– Instead of placing the numbers before the root name (1-butene), they recommended placing them immediately before the part of the name they locate (but-1-ene).
– The new placement is helpful for clarifying the names of compounds containing multiple functional groups.
– You should be prepared to recognize names using either placement of the numbers, because both are widely used.
– In this subject , names using the old number placement are printed in blue, and those using the new number placement are printed in green.
– A compound with two double bonds is a diene.
– A triene has three double bonds, and a tetraene has four.
– Numbers are used to specify the locations of the double bonds.
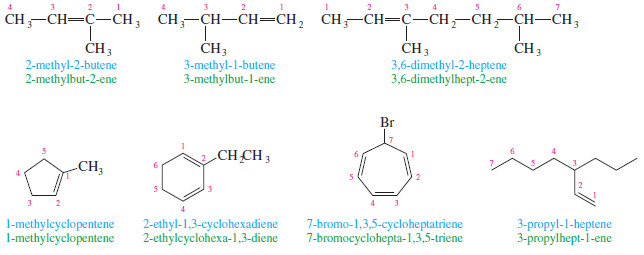
– Each alkyl group attached to the main chain is listed with a number to give its location.
– Note that the double bond is still given preference in numbering, however
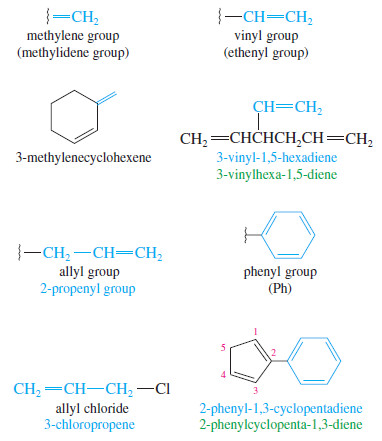
Alkenes as Substituents
– Alkenes named as substituents are called alkenyl groups.
– They can be named systematically (ethenyl, propenyl, etc.), or by common names.
– Common alkenyl substituents are the vinyl, allyl, methylene, and phenyl groups.
– The phenyl group (Ph) is different from the others because it is aromatic and does not undergo the typical reactions of alkenes.
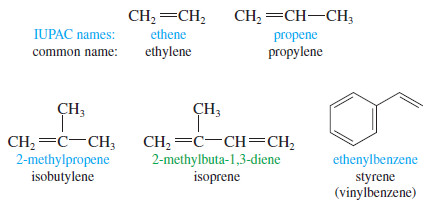
Common Names
– Most alkenes are conveniently named by the IUPAC system, but common names are sometimes used for the simplest compounds.
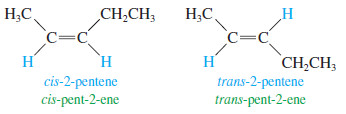
Cis-Trans Nomenclature of Alkenes
– the rigidity and lack of rotation of carbon–carbon double bonds give rise to cis-trans isomerism, also called geometric isomerism.
– If two similar groups bonded to the carbons of the double bond are on the same side of the bond, the alkene is the cis isomer.
– If the similar groups are on opposite sides of the bond, the alkene is trans.
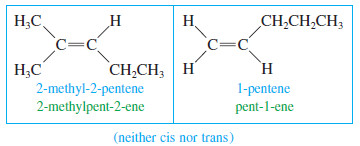
– Not all alkenes are capable of showing cis-trans isomerism.
– If either carbon of the double bond holds two identical groups, the molecule cannot have cis and trans forms.
– Following are some cis and trans alkenes and some alkenes that cannot showcis-trans isomerism.
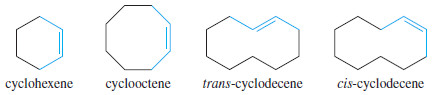
– Trans cycloalkenes are unstable unless the ring is large enough (at least eight carbon atoms) to accommodate the trans double bond.
– Therefore, all cycloalkenes are assumed to be cis unless they are specifically named trans.
– The cis name is rarely used with cycloalkenes, except to distinguish a large cycloalkene from its trans isomer.
E-Z Nomenclature of Alkenes
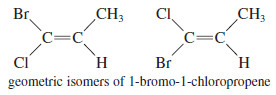
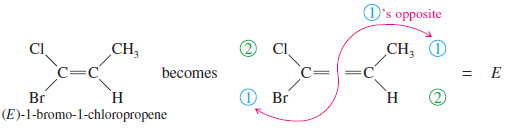
– The cis-trans nomenclature for geometric isomers sometimes gives an ambiguous name.
– For example, the isomers of 1-bromo-1-chloropropene are not clearly cis or trans because it is not obvious which substituents are referred to as being cis or trans.
– To deal with this problem, we use the E-Z system of nomenclature (pun intended) for cis-trans isomers, which is patterned after the Cahn–Ingold Prelog convention for asymmetric carbon atoms.
– It assigns a unique configuration of either E or Z to any double bond capable of geometric isomerism.
– To name an alkene by the E-Z system, mentally separate the double bond into its two ends.
– Remember how you used the Cahn–Ingold–Prelog rules to assign relative priorities to groups on an asymmetric carbon atom so you could name it (R) or (S).
– Consider each end of the double bond separately, and use those same rules to assign first and second priorities to the two substituent groups on that end. Do the same for the other end of the double bond.
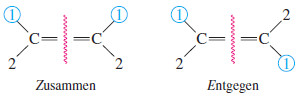
– If the two first-priority atoms are together (cis) on the same side of the double bond, you have the Z isomer, from the German word zusammen, “together.”
– If the two first-priority atoms are on opposite (trans) sides of the double bond, you have the E isomer, from the German word entgegen, “opposite.”
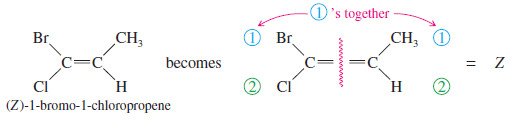
Example for E-Z Nomenclature
– The other isomer is named similarly:
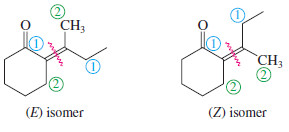
– The following example shows the use of the E-Z nomenclature with cyclic stereoisomers that are not clearly cis or trans.
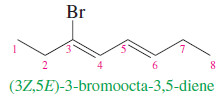
– If the alkene has more than one double bond, the stereochemistry about each double bond should be specified.
– The following compound is properly named (3Z,5E)-3-bromoocta-3,5-diene:
– The use of E-Z names (rather than cis and trans) is always an option, but it is required whenever a double bond is not clearly cis or trans.
– Most trisubstituted and tetrasubstituted double bonds are more clearly named E or Z rather than cis or trans.
SUMMARY: Rules for Naming Alkenes
The following rules summarize the IUPAC system for naming alkenes:
(1) Select the longest chain or largest ring that contains the largest possible number of double bonds, and name it with the -ene suffix.
If there are two double bonds, the suffix is -diene; for three, -triene; for four, -tetraene; and so on.
(2) Number the chain from the end closest to the double bond(s). Number a ring so that the double bond is between carbons 1 and 2.
Place the numbers giving the locations of the double bonds in front of the root name (old system) or in front of the suffix -ene, -diene, etc. (new system).
(3) Name substituent groups as in alkanes, indicating their locations by the number of the main-chain carbon to which they are attached.
The ethenyl group and the propenyl group are usually called the vinyl group and the allyl group, respectively.
(4) For compounds that show geometric isomerism, add the appropriate prefix: cis- or trans-, or E- or Z-.
Cycloalkenes are assumed to be cis unless named otherwise.