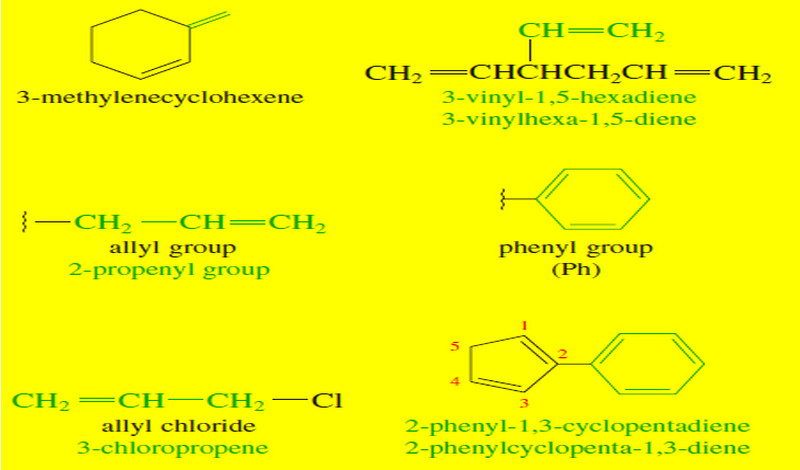
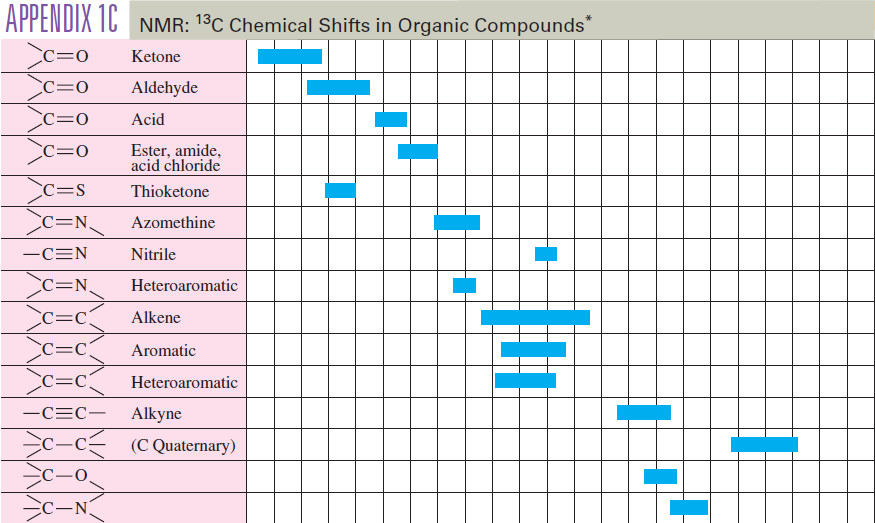
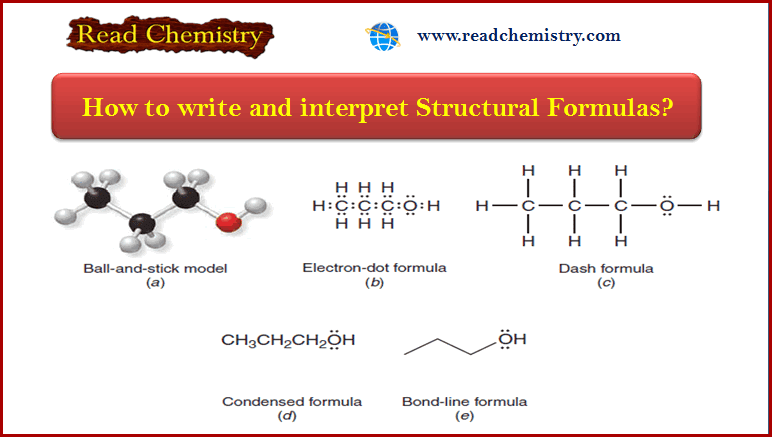
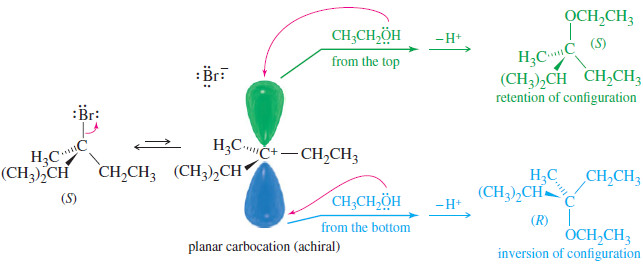
Common Uses of Alkyl Halides
Alkyl halides as Solvents
– Alkyl halides are used primarily as industrial and household solvents.
– Carbon tetrachloride (CCl4) was once used for dry cleaning, spot removing, and other domestic cleaning.
– Carbon tetrachloride is toxic and carcinogenic (causes cancer), however, so dry cleaners now use 1,1,1-trichloroethane and other solvents instead.
– Methylene chloride (CH2Cl2) and chloroform (CHCl3) are also good solvents for cleaning and degreasing work.
– Methylene chloride was once used to dissolve the caffeine from coffee beans to produce decaffeinated coffee.
– Concerns about the safety of coffee with residual traces of methylene chloride prompted coffee producers to use liquid carbon dioxide instead.
– Chloroform is more toxic and carcinogenic than methylene chloride; it has been replaced by methylene chloride and other solvents in most industrial degreasers and paint removers.
– Even the safest halogenated solvents, such as methylene chloride and 1,1,1- trichloroethane, should be used carefully.
– They are all potentially toxic and carcinogenic, and they dissolve the fatty oils that protect skin, causing a form of dermatitis.
Alkyl halides as Reagents
– Many syntheses use alkyl halides as starting materials for making more complex molecules.
– The conversion of alkyl halides to organometallic reagents (compounds containing carbon–metal bonds) is a particularly important tool for organic synthesis.
– We discuss the formation of organometallic compounds in Section 10-8.
Alkyl halides as Anesthetics
– In the 1840s, chloroform (CHCl3) was found to produce general anesthesia, opening new possibilities for careful surgery with a patient who is unconscious and relaxed.
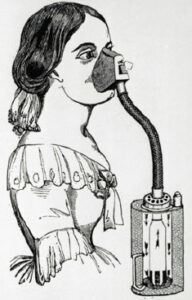
– Chloroform is toxic and carcinogenic, however, and it was soon abandoned in favor of safer anesthetics, such as diethyl ether.
– A less toxic halogenated anesthetic is a mixed alkyl halide (CF3CHClBr) , which goes by the trade name Halothane.
– Ethyl chloride is often used as a topical anesthetic for minor procedures.
– When sprayed on the skin, its evaporation (bp 12 °C) cools the area and enhances the numbing effect.
Freons: Refrigerants and Foaming Agents
– The freons (also called chlorofluorocarbons, or CFCs) are fluorinated haloalkanes that were developed to replace ammonia as a refrigerant gas.
– Ammonia is toxic, and leaking refrigerators often killed people who were working or sleeping nearby.
– Freon-12®, CF2Cl2, was at one time the most widely used refrigerant.
– Low-boiling freons (such as Freon-11®,CCl3F ) were once used as foaming agents that were added to a plastic to vaporize and form a froth that hardens into a plastic foam.
– The release of freons into the atmosphere has raised concerns about their reactions with the earth’s protective ozone layer.
– CFCs gradually diffuse up into the stratosphere, where the chlorine atoms catalyze the decomposition of ozone (O3) into oxygen(O2)
– Most scientists blame the freoncatalyzed depletion of ozone for the “hole” in the ozone layer that has been detected over the South Pole.
– International treaties have limited the future production and use of the ozonedestroying freons.
– Freon-12 has been replaced in aerosol cans by low boiling hydrocarbons or carbon dioxide.
– In refrigerators and automotive air conditioners, Freon-12 has been replaced by Freon-22®,CHClF2
– Freons with (C-H) bonds (such as Freon-22), called HCFCs, are generally destroyed at lower altitudes before they reach the stratosphere.
– Propane, CO2 and HCFC-123 (CHCl2CF3) are used as substitutes for Freon-11 in making plastic foams.
Pesticides
– Alkyl halides have contributed to human health through their use as insecticides.
– Since antiquity, people have died from famine and disease caused or carried by mosquitoes, fleas, lice, and other vermin.
– The “black death” of the Middle Ages wiped out nearly a third of the population of Europe through infection by the flea-borne bubonic plague.
– Whole regions of Africa and tropical America were uninhabited and unexplored because people could not survive insect-borne diseases such as malaria, yellow fever, and sleeping sickness.
– Arsenic compounds, nicotine, and other crude insecticides were developed in the nineteenth century, but these compounds are just as toxic to birds, animals, and people as they are to insects. Their use is extremely hazardous, but a hazardous insecticide was still preferable to certain death by disease or starvation.
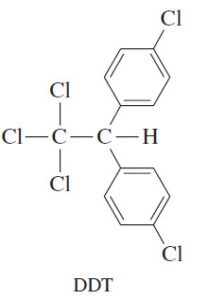
– The war against insects changed dramatically in 1939 with the discovery of DDT (Figure 6-2).
– DDT is extremely toxic to insects, but its toxicity in mammals is quite low.
– About an ounce of DDT is required to kill a person, but that same amount of insecticide protects an acre of land against locusts or mosquitoes. In 1970, the U.S. National Academy of Sciences reported, “in little more than two decades DDT has prevented 500 million deaths due to malaria.”
– Similar advances were made against the mosquitoes carrying yellow fever and the tsetse flies carrying sleeping sickness.
– Using DDT as a body dust protected people against louse-borne typhus, and dusting rodent burrows controlled the threat of plague.
– As with many inventions, DDT showed undesired side effects.
– It is a long-lasting insecticide, and its residues accumulate in the environment.
– The widespread use of DDT as an agricultural insecticide led to the development of substantial DDT concentrations in wildlife, causing declines in several species.
– In 1972, DDT was banned by the U.S. Environmental Protection Agency for use as an agricultural insecticide.
– It is still used, however, in places where insect-borne diseases threaten human life.
– DDT-treated bed netting is still the most cost-effective protection against malaria, and careful spraying of DDT around dwellings and in rodent burrows has helped to control the spread of deadly diseases.
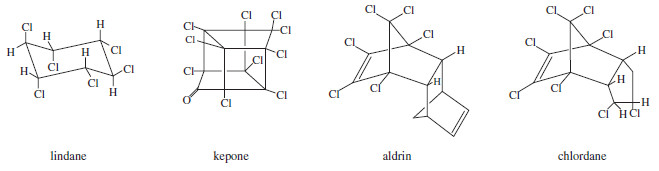
– Many other chlorinated insecticides have been developed.
– Some of them also accumulate in the environment, gradually producing toxic effects in wildlife.
– Others can be used with little adverse impact if they are applied properly.
– Because of their persistent toxic effects, chlorinated insecticides are rarely used in agriculture.
– They are generally used when a potent insecticide is needed to protect life or property.
– For example, lindane is used in shampoos to kill lice, and chlordane is used to protect wooden buildings from termites.
– The structures of some chlorinated insecticides are shown .