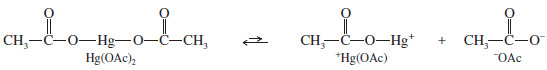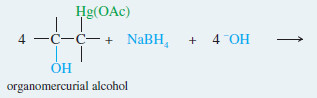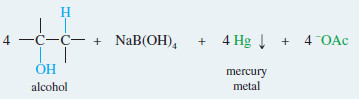Oxymercuration–demercuration of alkenes
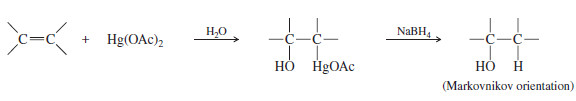
– Oxymercuration–demercuration of alkenes is another method for converting alkenes to alcohols with Markovnikov orientation.
Hydration of alkenes by Oxymercuration–Demercuration
– Many alkenes do not easily undergo hydration in aqueous acid.
– Some alkenes are nearly insoluble in aqueous acid, and others undergo side reactions such as rearrangement, polymerization, or charring under these strongly acidic conditions.
– In some cases, the overall equilibrium favors the alkene rather than the alcohol.
– No amount of catalysis can cause a reaction to occur if the energetics are unfavorable.
– Oxymercuration–demercuration is another method for converting alkenes to alcohols with Markovnikov orientation.
– Oxymercuration demercuration works with many alkenes that do not easily undergo direct hydration, and it takes place under milder conditions.
– No free carbocation is formed, so there is no opportunity for rearrangements or polymerization.
– The reagent for mercuration is mercuric acetate Hg(OCOCH3)2, abbreviated Hg(OAc)2
– There are several theories as to how this reagent acts as an electrophile; the simplest one is that mercuric acetate dissociates slightly to form a positively charged mercury species, +Hg(OAc).
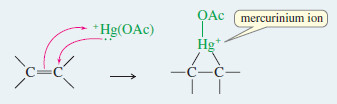
– Oxymercuration involves an electrophilic attack on the double bond by the positively charged mercury species.
– The product is a mercurinium ion, an organometallic cation containing a three-membered ring.
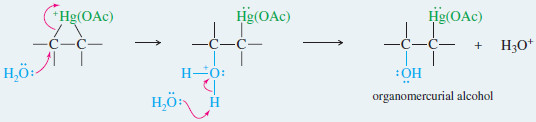
– In the second step, water from the solvent attacks the mercurinium ion to give (after deprotonation) an organomercurial alcohol.
– A subsequent reaction is demercuration, to remove the mercury.
– Sodium borohydride (NaBH4 a reducing agent) replaces the mercuric acetate fragment with a hydrogen atom.
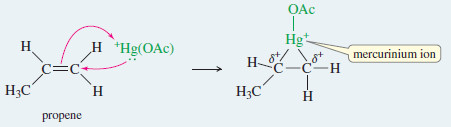
Mechanism: Oxymercuration of an Alkene
Step 1: Electrophilic attack forms a mercurinium ion.
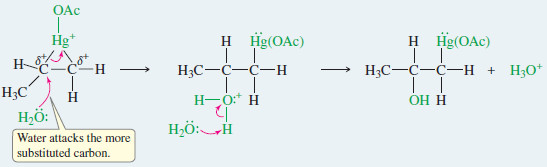
Step 2: Water opens the ring to give an organomercurial alcohol.
Demercuration replaces the mercuric fragment with hydrogen to give the alcohol.
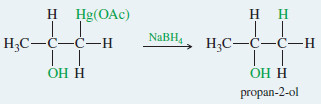
Example: Oxymercuration–demercuration of propene.
Step 1: Electrophilic attack forms a mercurinium ion
Step 2: Water opens the ring to give an organomercurial alcohol.
Demercuration replaces the mercuric fragment with hydrogen to give the alcohol.
Oxymercuration–demercuration of an unsymmetrical alkene
– Oxymercuration–demercuration of an unsymmetrical alkene generally gives Markovnikov orientation of addition, as shown by the oxymercuration of propene in the preceding example.
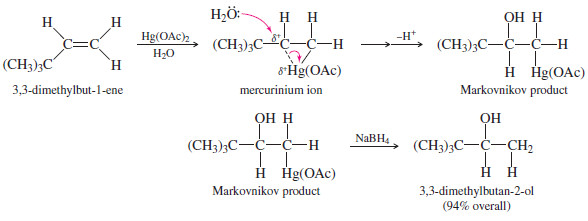
– The mercurinium ion has a considerable amount of positive charge on both of its carbon atoms, but there is more of a positive charge on the more substituted carbon atom, where it is more stable.
– Attack by water occurs on this more electrophilic carbon, giving Markovnikov orientation.
– The electrophile, +Hg(OAc), remains bonded to the less substituted end of the double bond.
– Reduction of the organomercurial alcohol gives the Markovnikov alcohol: propan-2-ol.
– Similarly, oxymercuration–demercuration of 3,3-dimethylbut-1-ene gives the Markovnikov product, 3,3-dimethylbutan-2-ol, in excellent yield.
– Contrast this unrearranged product with the rearranged product formed in the acid-catalyzed hydration of the same alkene .
– Oxymercuration–demercuration reliably adds water across the double bond of an alkene with Markovnikov orientation and without rearrangement.
– Of the methods we have seen for Markovnikov hydration of alkenes, oxymercuration–demercuration is most commonly used in the laboratory.
– It gives better yields than direct acid-catalyzed hydration, it avoids the possibility of rearrangements, and it does not involve harsh conditions.
– There are also disadvantages, however. Organomercurial compounds are highly toxic.
– They must be used with great care and then must be disposed of properly.
Alkoxymercuration–demercuration
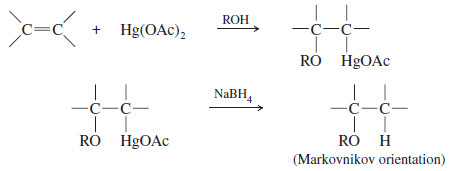
– When mercuration takes place in an alcohol solvent, the alcohol serves as a nucleophile to attack the mercurinium ion.
– The resulting product contains an alkoxy (-O-R)group. In effect, alkoxymercuration–demercuration converts alkenes to ethers by adding an alcohol across the double bond of the alkene
– As we have seen, an alkene reacts to form a mercurinium ion that is attacked by the nucleophilic solvent.
– Attack by an alcohol solvent gives an organomercurial ether that can be reduced to the ether.
– The solvent attacks the mercurinium ion at the more substituted end of the double bond (where there is more δ+ charge), giving Markovnikov orientation of addition.
– The Hg(OAc) group appears at the less substituted end of the double bond.
– Reduction gives the Markovnikov product, with hydrogen at the less substituted end of the double bond.
Solved problem
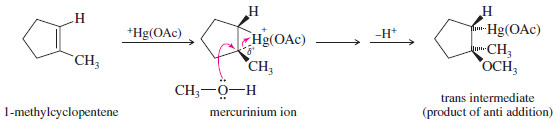
Show the intermediates and products that result from alkoxymercuration demercuration of 1-methylcyclopentene, using methanol as the solvent.
Solution
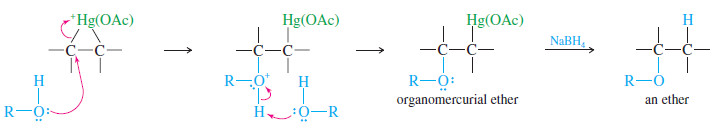
– Mercuric acetate adds to 1-methylcyclopentene to give the cyclic mercurinium ion.
– This ion has a considerable amount of positive charge on the more substituted tertiary carbon atom.
– Methanol attacks this carbon from the opposite side, leading to anti addition: The reagents (HgOAc and OCH3) have added to opposite faces of the double bond.
Reduction of the intermediate gives the Markovnikov product, 1-methoxy-1-methylcyclopentane.