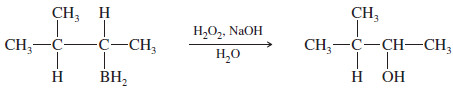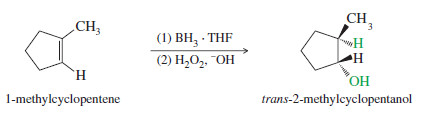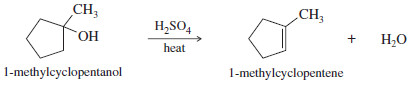Hydroboration of Alkenes
Hydroboration of Alkenes
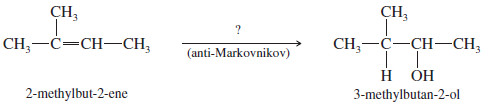
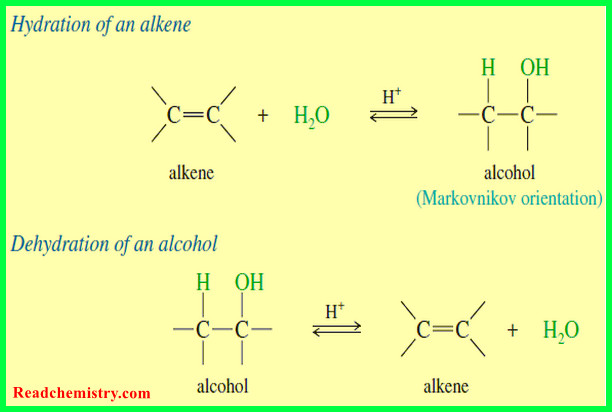
– We have seen two methods for hydrating an alkene with Markovnikov orientation.
– What if we need to convert an alkene to the anti-Markovnikov alcohol?
– For example, the following transformation cannot be accomplished using the hydration procedures covered thus far.
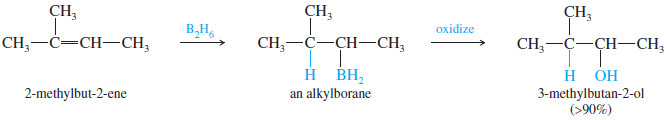
– Such an anti-Markovnikov hydration was impossible until H. C. Brown, of Purdue University, discovered that diborane (B2H6) adds to alkenes with anti-Markovnikov orientation to form alkylboranes, which can be oxidized to give anti-Markovnikov alcohols.
– This discovery led to the development of a large field of borane chemistry, for which Brown received the Nobel Prize in Chemistry in 1979.
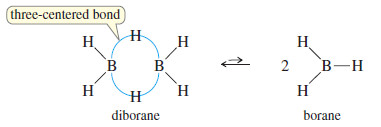
– Diborane (B2H6) is a dimer composed of two molecules of borane (B2H6).
– The bonding in diborane is unconventional, using three-centered (banana-shaped) bonds with protons in the middle of them.
– Diborane is in equilibrium with a small amount of borane (BH3) a strong Lewis acid with only six valence electrons.
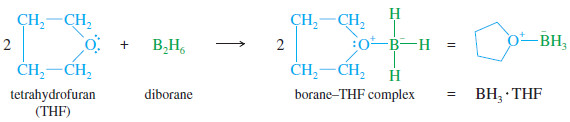
– Diborane is an inconvenient reagent. It is a toxic, flammable, and explosive gas.
– It is more easily used as a complex with tetrahydrofuran (THF), a cyclic ether.
– This complex reacts like diborane, yet the solution is easily measured and transferred.
– The BH3 , THF reagent is the form of borane commonly used in organic reactions.
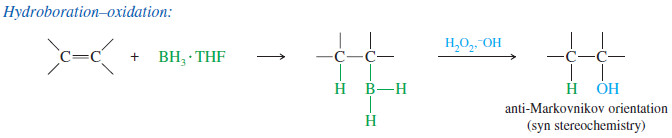
– BH3 adds to the double bond of an alkene to give an alkylborane. Basic hydrogen peroxide oxidizes the alkylborane to an alcohol.
– In effect, hydroboration–oxidation converts alkenes to alcohols by adding water across the double bond, with anti-Markovnikov orientation
(A) Mechanism of Hydroboration of Alkenes
– Borane is an electron-deficient compound. It has only six valence electrons, so the boron
atom in BH3 cannot have an octet.
– Acquiring an octet is the driving force for the unusual bonding structures (“banana” bonds, for example) found in boron compounds.
– As an electron-deficient compound, BH3 is a strong electrophile, capable of adding to a double bond.
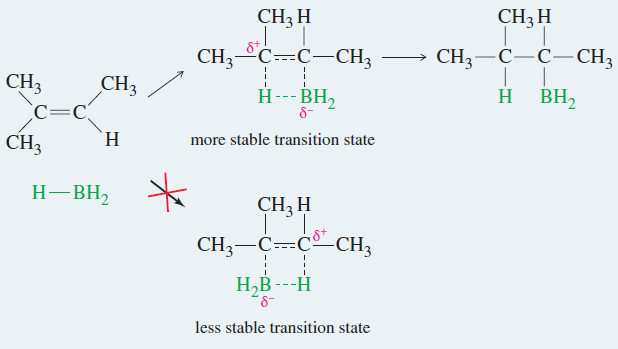
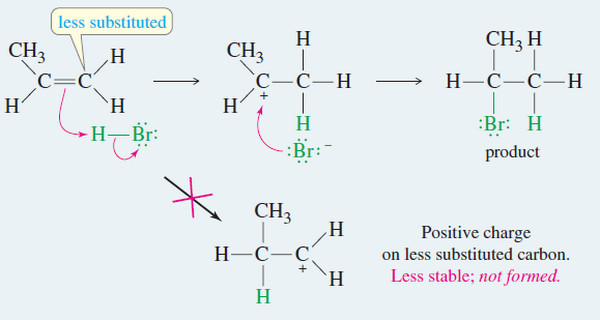
– This hydroboration of the double bond is thought to occur in one step, with the boron atom adding to the less substituted end of the double bond, as shown in Mechanism below.
– In the transition state, the electrophilic boron atom withdraws electrons from the pi bond, and the carbon at the other end of the double bond acquires a partial positive charge. This partial charge is more stable on the more substituted carbon atom.
– The product shows boron bonded to the less substituted end of the double bond and hydrogen bonded to the more substituted end.
– Also, steric hindrance favors boron adding to the less hindered, less substituted end of the double bond.
Mechanism: Hydroboration of an Alkene
– Borane adds to the double bond in a single step.
– Boron adds to the less hindered, less substituted carbon, and hydrogen adds to the more substituted carbon.
– The boron atom is removed by oxidation, using aqueous sodium hydroxide and hydrogen peroxide (HOOH or H2O2) to replace the boron atom with a hydroxyl ( -OH) group.
– The oxidation does not affect the orientation of the product, because the anti- Markovnikov orientation was established in the first step, the addition of BH3.
– This hydration of an alkene by hydroboration–oxidation is another example of a reaction that does not follow the original statement of Markovnikov’s rule (the product is anti-Markovnikov), but still follows our understanding of the reasoning behind Markovnikov’s rule.
– The electrophilic boron atom adds to the less substituted end of the double bond, placing the positive charge (and the hydrogen atom) at the more substituted end.
Solved problem
Show how you would convert 1-methylcyclopentanol to 2-methylcyclopentanol.
Solution
– Working backward, use hydroboration–oxidation to form 2-methyl-cyclopentanol from 1-methylcyclopentene.
– The use of (1) and (2) above and below the reaction arrow indicates individual steps in a two-step sequence.
– The 2-methylcyclopentanol that results from this synthesis is the pure trans isomer.
– 1-Methylcyclopentene is the most substituted alkene that results from dehydration of 1-methylcyclopentanol.
– Dehydration of the alcohol would give the correct alkene.
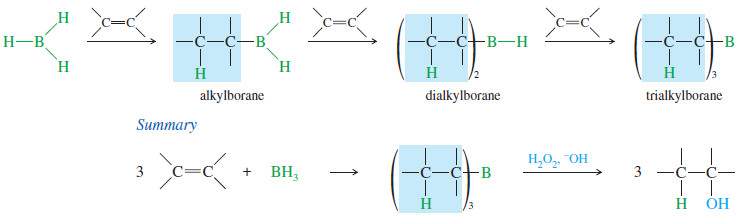
(B) Stoichiometry of Hydroboration of Alkenes
– For simplicity, we have neglected the fact that 3 moles of an alkene can react with each mole
of BH3.
– Each B-H bond in BH3 can add across the double bond of an alkene.
– The first addition forms an alkylborane, the second a dialkylborane, and the third a trialkylborane
– Trialkylboranes react exactly as we have discussed, and they oxidize to give anti- Markovnikov alcohols.
– Trialkylboranes are quite bulky, further reinforcing the preference for boron to add to the less hindered carbon atom of the double bond.
– Boranes are often drawn as the 1:1 monoalkylboranes to simplify their structure and emphasize the organic part of the molecule.
(C) Stereochemistry of Hydroboration of Alkenes
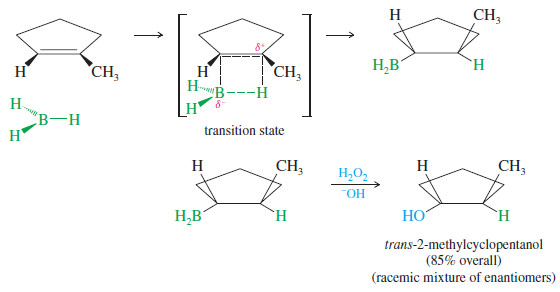
– The simultaneous addition of boron and hydrogen to the double bond (as shown in Mechanism above) leads to a syn addition: Boron and hydrogen add across the double bond on the same side of the molecule. (If they added to opposite sides of the molecule, the process would be an anti addition.)
– The stereochemistry of the hydroboration–oxidation of 1-methylcyclopentene is shown next.
– Boron and hydrogen add to the same face of the double bond (syn) to form a trialkylborane.
– Oxidation of the trialkylborane replaces boron with a hydroxyl group in the same stereochemical position. The product is trans-2-methylcyclopentanol.
– A racemic mixture is expected because a chiral product is formed from achiral reagents
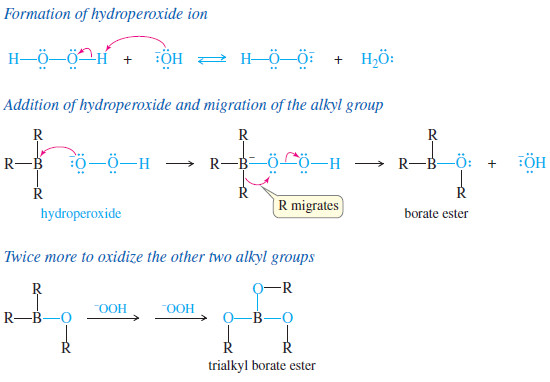
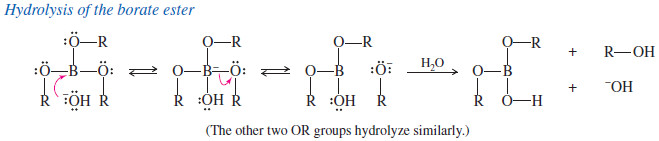
– The second step (oxidation of the borane to the alcohol) takes place with retention of configuration.
– Hydroperoxide ion adds to the borane, causing the alkyl group to migrate from boron to oxygen.
– The alkyl group migrates with retention of configuration because it moves with its electron pair and does not alter the tetrahedral structure of the migrating carbon atom.
– Hydrolysis of the borate ester gives the alcohol.
– Hydroboration of alkenes is another example of a stereospecific reaction, in which different stereoisomers of the starting compound react to give different stereoisomers of the product.
Solved problem
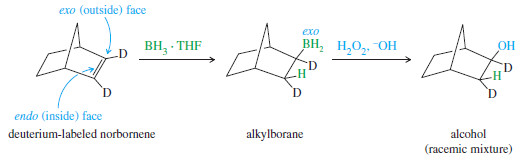
A norbornene molecule labeled with deuterium is subjected to hydroboration–oxidation. Give the structures of the intermediates and products.
Solution
– The syn addition of BH3 across the double bond of norbornene takes place mostly from the more accessible outside (exo) face of the double bond.
– Oxidation gives a product with both the hydrogen atom and the hydroxyl group in exo positions. (The less accessible inner face of the double bond is called the endo face.)