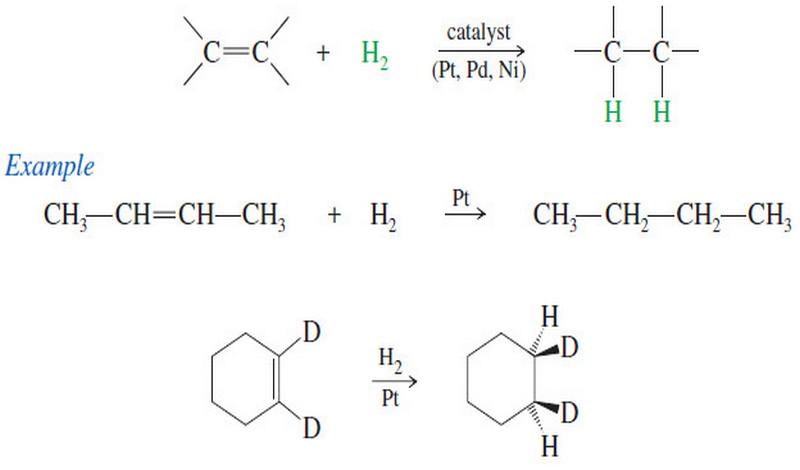
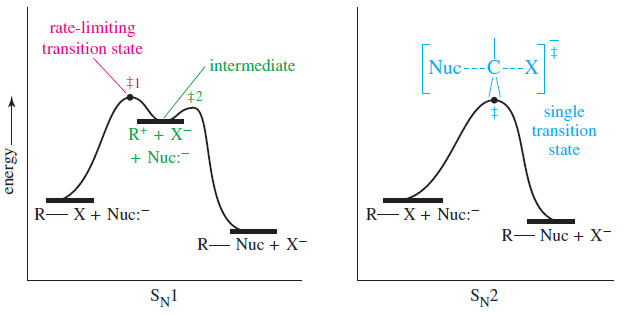
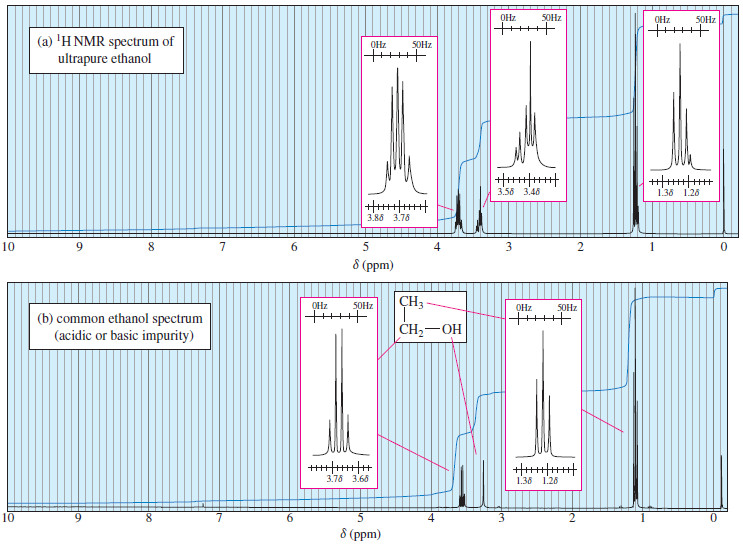
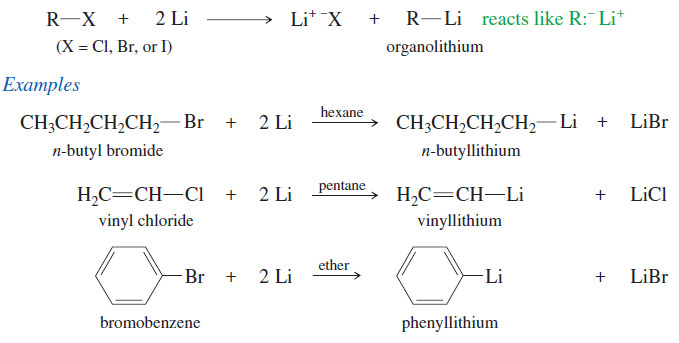
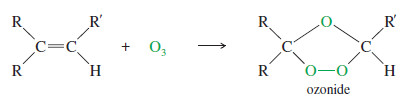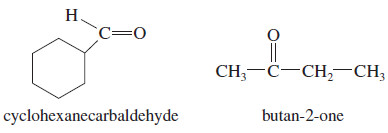Oxidative Cleavage of Alkenes
Oxidative Cleavage of Alkenes
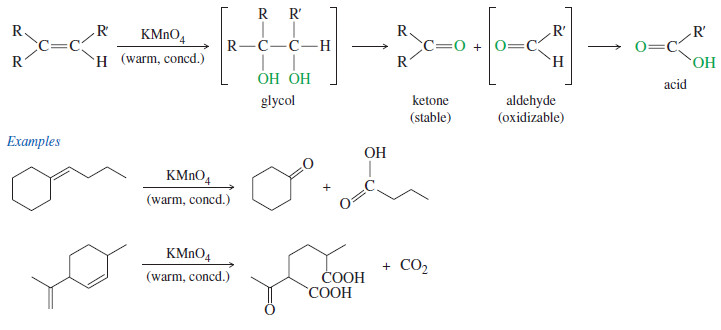
Cleavage by Permanganate
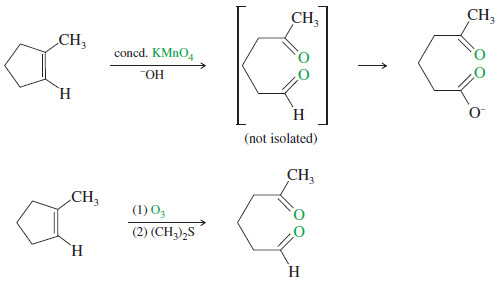
– In a potassium permanganate dihydroxylation, if the solution is warm or acidic or too concentrated, oxidative cleavage of the glycol may occur.
– In effect, the double bond is cleaved to two carbonyl groups.
– The products are initially ketones and aldehydes, but aldehydes are oxidized to carboxylic acids under these strong oxidizing conditions.
– If the molecule contains a terminal =CH2 group, that group is oxidized all the way to CO2 and water.
Ozonolysis
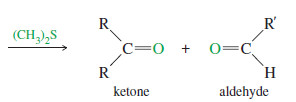
– Like permanganate, ozone cleaves double bonds to give ketones and aldehydes. However, ozonolysis is milder, and both ketones and aldehydes can be recovered without further oxidation.
– Ozone (O3) is a high-energy form of oxygen produced when ultraviolet light or an electrical discharge passes through oxygen gas.
– Ultraviolet light from the sun converts oxygen to ozone in the upper atmosphere.
– This “ozone layer” shields the earth from some of the high-energy ultraviolet radiation it would otherwise receive.
– Ozone has 142 kJ mol of excess energy over oxygen, and it is much more reactive.
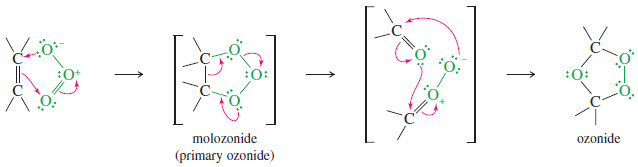
– A Lewis structure of ozone shows that the central oxygen atom bears a positive charge, and each of the outer oxygen atoms bears half a negative charge.
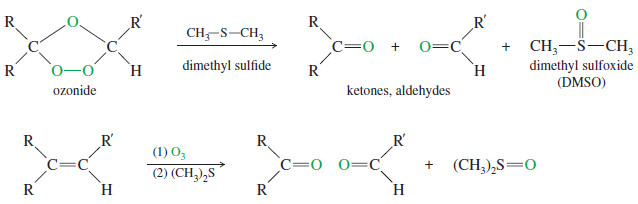
– Ozone reacts with an alkene to form a cyclic compound called a primary ozonide or molozonide (because 1 mole of ozone has been added).
– The molozonide has two peroxy (-O-O-) linkages, so it is quite unstable.
– It rearranges rapidly, even at low temperatures, to form an ozonide
– Ozonides are not very stable, and they are rarely isolated.
– In most cases, they are immediately reduced by a mild reducing agent such as zinc or (more recently) dimethyl sulfide.
– The products of this reduction are ketones and aldehydes.
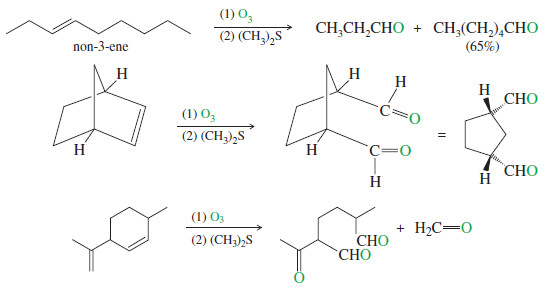
– The following reactions show the products obtained from ozonolysis of some representative alkenes.
– Note how (1) and (2) are used with a single reaction arrow to denote the steps in a two-step sequence.
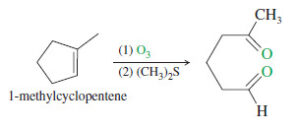
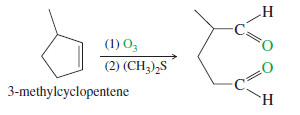
– One of the most common uses of ozonolysis has been for determining the positions of double bonds in alkenes.
– For example, if we were uncertain of the position of the methyl group in a methylcyclopentene, the products of ozonolysis–reduction would confirm the structure of the original alkene.
Solved problem
Ozonolysis–reduction of an unknown alkene gives an equimolar mixture of cyclohexanecarbaldehyde and butan-2-one. Determine the structure of the original alkene
Solution:
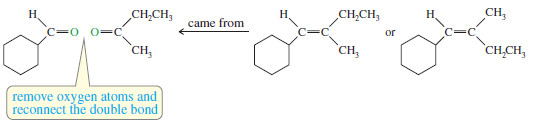
We can reconstruct the alkene by removing the two oxygen atoms of the carbonyl groups (C=O) and connecting the remaining carbon atoms with a double bond.
One uncertainty remains, however: The original alkene might be either of two possible geometric isomers.
Comparison of Permanganate Cleavage and Ozonolysis
– Both permanganate and ozonolysis break the carbon–carbon double bond and replace it with carbonyl groups.
In the permanganate cleavage, any aldehyde products are further oxidized to carboxylic acids.
In the ozonolysis–reduction procedure, the aldehyde products are generated in the dimethyl sulfide reduction step (and not in the presence of ozone), and they are not oxidized