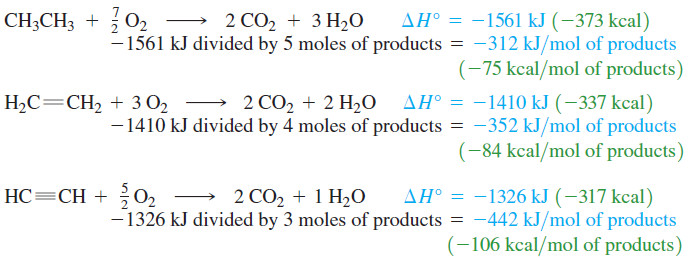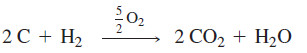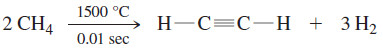Importance of Alkynes : Acetylene
Commercial Importance of Alkynes : Acetylene and Methylacetylene
Uses of Acetylene and Methylacetylene
– Acetylene is by far the most important commercial alkyne.
– and Acetylene is an important industrial feedstock, but its largest use is as the fuel for the oxyacetylene welding torch.
– It is a colorless, foul-smelling gas that burns in air with a yellow, sooty flame.

– When the flame is supplied with pure oxygen, however, the color turns to light blue, and the flame temperature increases dramatically.
– A comparison of the heat of combustion for acetylene with those of ethene and ethane shows this gas makes an excellent fuel for a high-temperature flame.
– If we were simply heating a house by burning one of these fuels, we might choose ethane as our fuel because it produces the most heat per mole of gas consumed.
– In the welding torch, we want the highest possible temperature of the gaseous products.
– The heat of reaction must raise the temperature of the products to the flame temperature.
– Roughly speaking, the increase in temperature of the products is proportional to the heat given off per mole of products formed.
– This rise in temperature is largest with acetylene, which gives off the most heat per mole of products.
– The oxyacetylene flame reaches temperatures as high as 2800 °C.
– When acetylene was first used for welding, it was considered a dangerous, explosive gas.
– Acetylene is thermodynamically unstable.
– When the compressed gas is subjected to thermal or mechanical shock, it decomposes to its elements, releasing 234 kJ (56 kcal) of energy per mole.
– This initial decomposition often splits the container, allowing the products (hydrogen and finely divided carbon) to burn in the air.
– Acetylene is safely stored and handled in cylinders that are filled with crushed firebrick wet with acetone.
– Acetylene dissolves freely in acetone, and the dissolved gas is not so prone to decomposition.
– Firebrick helps to control the decomposition by minimizing the free volume of the cylinder, cooling and controlling any decomposition before it gets out of control.
– and Methylacetylene also is used in welding torches.
– Methylacetylene does not decompose as easily as acetylene, and it burns better in air (as opposed to pure oxygen).
– Methylacetylene is well suited for household soldering and brazing that requires higher temperatures than propane torches can reach.
– The industrial synthesis of methylacetylene gives a mixture with its isomer, propadiene (allene).
– This mixture is sold commercially under the name MAPP® gas (MethylAcetylene-ProPadiene).
(2) Manufacture of Acetylene
– Acetylene, one of the cheapest organic chemicals, is made from coal or from natural gas.
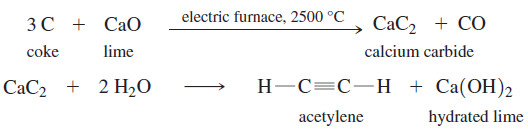
– The synthesis from coal involves heating lime and coke (roasted coal) in an electric furnace to produce calcium carbide.
– Addition of water to calcium carbide produces acetylene and hydrated lime.
– This second reaction once served as a light source in coal mines until batterypowered lights became available.
– A miner’s lamp works by allowing water to drip slowly onto some calcium carbide.

– Acetylene is generated, feeding a small flame where the gas burns in air with a yellow flickering light.
– Unfortunately, this flame ignites the methane gas commonly found in coal seams, causing explosions.
– Battery-powered miner’s lamps provide better light and reduce the danger of methane explosions.
– The synthesis of acetylene from natural gas is a simple process. Natural gas consists mostly of methane, which forms acetylene when it is heated for a very short period of time.
– Although this reaction is endothermic, there are twice as many moles of products as reactants.
– The increase in the number of moles results in an increase in entropy, and the (-TΔS) term in the free energy (ΔG = ΔH – TΔS) predominates at this high temperature.