The electronic structure of Alkynes
– we studied theThe electronic structureof Alkynes (a triple bond) in This subject: The Structure of Ethyne (Acetylene): sp Hybridization
– Let’s review The electronic structureof Alkynes, using acetylene as the example.
– The Lewis structure of acetylene shows three pairs of electrons in the region between the carbon nuclei:
– Each carbon atom is bonded to two other atoms, and there are no nonbonding valence electrons.
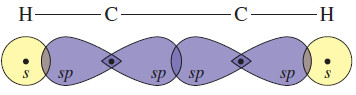
– Each carbon atom needs two hybrid orbitals to form the sigma bond framework.
– Hybridization of the s orbital with one p orbital gives two hybrid orbitals, directed 180° apart, for each carbon atom.
– Overlap of these sp hybrid orbitals with each other and with the hydrogen s orbitals gives the sigma bond framework.
– Experimental results have confirmed this linear (180°) structure.
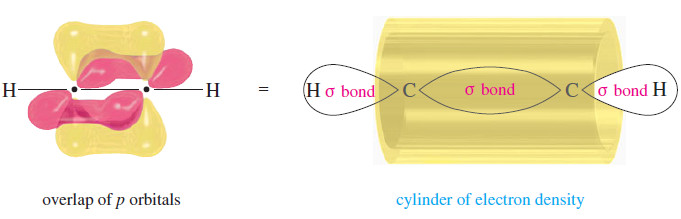
– Two pi bonds result from overlap of the two remaining unhybridized p orbitals on each carbon atom.
– These orbitals overlap at right angles to each other, forming one pi bond with electron density above and below the C-C sigma bond, and the other with electron density in front and in back of the sigma bond.
– The shape of these pi bonds is such that they blend to form a cylinder of electron density encircling the sigma bond between the two carbon atoms.
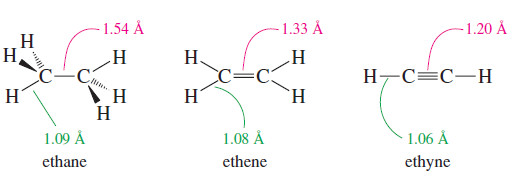
– The carbon–carbon bond length in acetylene is 1.20 Å, and each carbon–hydrogen bond is 1.06 Å.
– Both bonds are shorter than the corresponding bonds in ethane and in ethene.
– The triple bond is relatively short because of the attractive overlap of three bonding pairs of electrons and the high s character of the sp hybrid orbitals.
– The sp hybrid orbitals are about one-half (s) character (as opposed to one-third (s) character of sp2 hybrids and one-fourth of sp3 hybrids), using more of the closer, tightly held s orbital.
– The sp hybrid orbitals also account for the slightly shorter (C-H) bonds in acetylene compared with ethylene.