Oxidation states of Alcohols and Related Functional Groups
Oxidation states of Alcohols and Related Functional Groups
– Oxidation states of Alcohols leads to ketones, aldehydes, and carboxylic acids.
– These functional groups, in turn, undergo a wide variety of additional reactions.
– For these reasons, alcohol oxidations are some of the most common organic reactions.
– In inorganic chemistry, we think of Oxidation as a loss of electrons and Reduction as a gain of electrons.
– This picture works well for inorganic ions, as when Cr6+ is reduced to Cr3+
– Most organic compounds are uncharged, however, and gain or loss of electrons is not obvious.
– Organic chemists tend to think of oxidation as the result of adding an oxidizing agent ( O2, Br2, etc.), and reduction as the result of adding a reducing agent (H2, NaBH4, etc.).
Difference between Oxidation and Reduction
– Most organic chemists habitually use the following simple rules, based on the change in the formula of the substance:
Oxidation: addition of O or O2 – addition of X2 (halogens) – loss of H2
Reduction: addition of H2 (or H–) – loss of O or O2 – loss of X2
Neither: addition or loss of H+, –OH , H2O, HX, etc. is neither an oxidation nor a reduction.
Oxidation of Alcohols
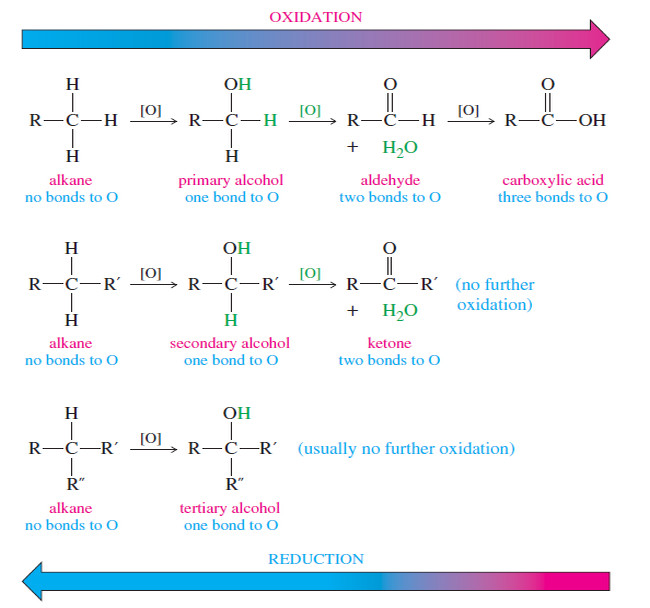
– We can tell that an oxidation or a reduction of an alcohol has taken place by counting the number of C-O bonds to the carbon atom.
– Oxidation usually converts C-H bonds to C-O bonds.
– The first row of structures in the Figure below shows that a primary alcohol is more oxidized than an alkane because the carbinol (C-OH) carbon atom has one bond to oxygen, while the alkane has no bonds to oxygen.
– Oxidation of a primary alcohol gives an aldehyde with a carbonyl carbon having two bonds to oxygen.
– Oxidation of the aldehyde to an acid adds another bond to oxygen, for a total of three.
– Further oxidation would require breaking a carbon–carbon bond to give four bonds to oxygen, the oxidation state of carbon dioxide.
– The following Figure compares the oxidation states of primary, secondary, and tertiary alcohols with those obtained by oxidation or reduction.
– The symbol [O] indicates an unspecified oxidizing agent.
– Notice that oxidation of a primary or secondary alcohol forms a carbonyl group by the removal of two hydrogen atoms: one from the carbinol carbon and one from the hydroxyl group.
– A tertiary alcohol cannot easily oxidize because there is no hydrogen atom available on the carbinol carbon.
Summary: Oxidation states of alcohols
– An alcohol is more oxidized than an alkane, yet less oxidized than carbonyl compounds such as ketones, aldehydes, and acids.
– Oxidation of a primary alcohol leads to an aldehyde, and further oxidation leads to an acid.
– Secondary alcohols are oxidized to ketones.
– Tertiary alcohols cannot be oxidized without breaking carbon–carbon bonds