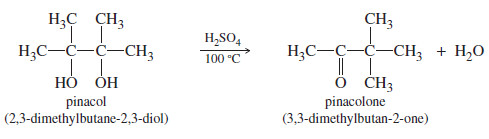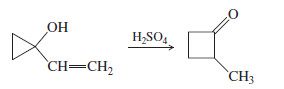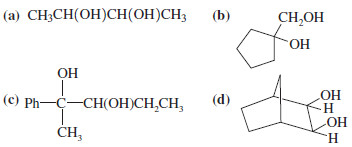Reactions of Diols
Unique Reactions of Diols
– Unique Reactions of Diols are:
(1) The Pinacol Rearrangement
(2) Periodic Acid Cleavage of Glycols
(1) The Pinacol Rearrangement
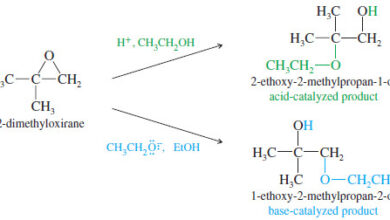
– Using our knowledge of alcohol reactions, we can explain results that seem strange at first glance.
– The following dehydration is an example of the pinacol rearrangement
– The pinacol rearrangement is formally a dehydration.
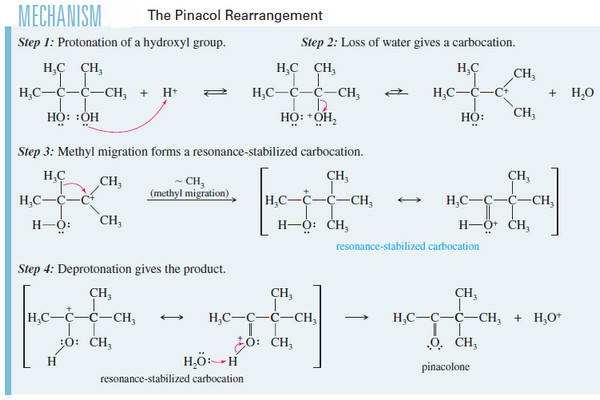
– The reaction is acid-catalyzed, the first step is protonation of one of the hydroxyl oxygens.
– Loss of water gives a tertiary carbocation, as expected for any tertiary alcohol.
– Migration of a methyl group places the positive charge on the carbon atom bearing the second group, where oxygen’s nonbonding electrons help to stabilize the positive charge through resonance.
This extra stability is the driving force for the rearrangement, which converts a relatively stable 3° carbocation into an even better resonancestabilized carbocation.
– Deprotonation of the resonance-stabilized cation gives the product, pinacolone.
– Pinacol-like rearrangements are common in acid-catalyzed reactions of diols.
– One of the hydroxyl groups protonates and leaves as water, forming a carbocation.
– Rearrangement gives a resonance-stabilized cation with the remaining hydroxyl group helping to stabilize the positive charge.
Problem (1)
– The following reaction involves a starting material with a double bond and a hydroxyl group, yet its mechanism resembles a pinacol rearrangement. Propose a mechanism, and point out the part of your mechanism that resembles a pinacol rearrangement.
Problem-solving Hint
By analogy with the pinacol rearrangement, watch for carbocation rearrangements that
the + charge to a carbinol carbon atom. Rearrangements often move a + charge to any carbon atom that is bonded to an oxygen or nitrogen atom having lone pairs that help to stabilize the positive charge
(2) Periodic Acid Cleavage of Glycols
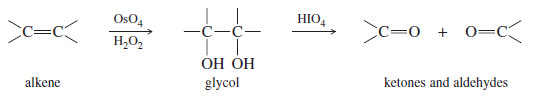
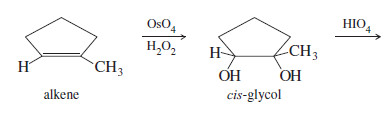
– 1,2-Diols (glycols), such as those formed by dihydroxylation of alkenes, are cleaved by periodic acid The products are the same ketones and aldehydes that would be formed by ozonolysis–reduction of the alkene.
– Dihydroxylation followed by periodic acid cleavage serves as a useful alternative to ozonolysis, and the periodate cleavage by itself is useful for determining the structures of sugars.
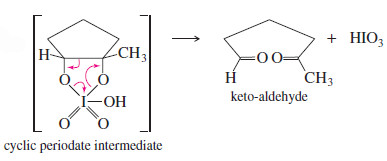
Periodic acid cleavage of a glycol probably involves a cyclic periodate intermediate likethat shown here.
Problem (2)
Predict the products formed by periodic acid cleavage of the following diols.
Problem-solving Hint
– Periodic acid cleaves a diol to give the same products as ozonolysis–reduction (O3 followed by Me2S) of the alkene.