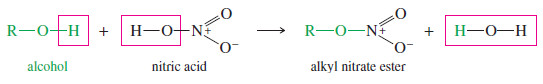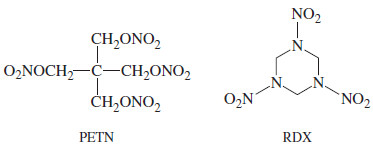Inorganic Esters – Esters of Inorganic Acids
Esters of Inorganic Acids
– In addition to forming esters with carboxylic acids, alcohols form inorganic esters with inorganic acids such as nitric acid, sulfuric acid, and phosphoric acid.
– In each type of ester, the alkoxy (-OR) group of the alcohol replaces a hydroxyl group of the acid, with loss of water.
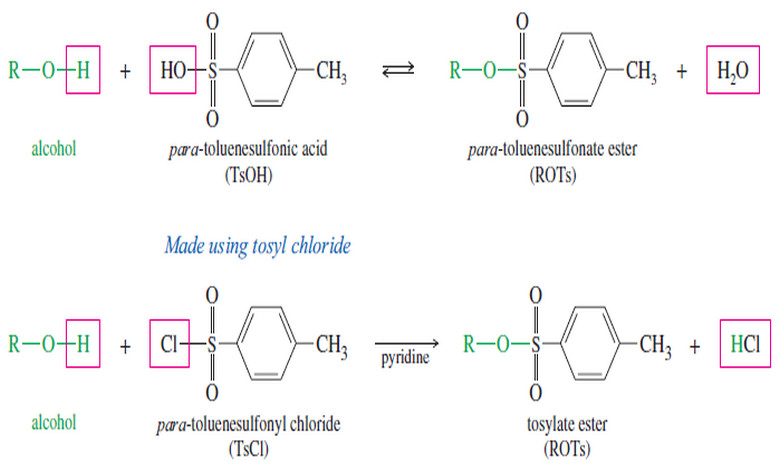
– We have already studied tosylate esters, composed of paratoluenesulfonic acid and alcohols (but made using tosyl chloride).
– Tosylate esters are analogous to sulfate esters , which are composed of sulfuric acid and alcohols.
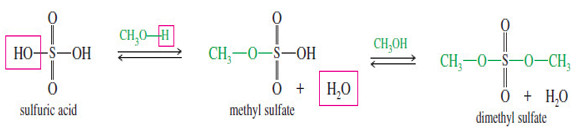
Sulfate Esters
– A sulfate ester is like a sulfonate ester, except there is no alkyl group directly bonded to the sulfur atom.
– In an alkyl sulfate ester, alkoxy groups are bonded to sulfur through oxygen atoms. Using methanol as the alcohol,
– Sulfate ions are excellent leaving groups. Like sulfonate esters, sulfate esters are good electrophiles.
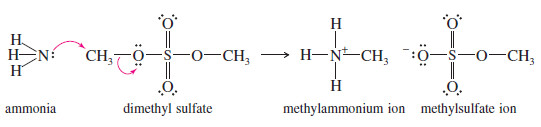
– Nucleophiles react with sulfate esters to give alkylated products.
– For example, the reaction of dimethyl sulfate with ammonia gives a sulfate salt of methylamine, CH3NH3+ CH3OSO3–
Nitrate Esters
– Nitrate esters are formed from alcohols and nitric acid.
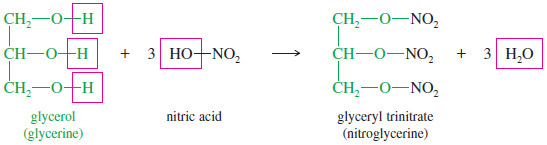
– The best-known nitrate ester is “nitroglycerine,” whose systematic name is glyceryl trinitrate.
– Glyceryl trinitrate results from the reaction of glycerol (1,2,3-propanetriol) with three molecules of nitric acid.
– First made in 1847, nitroglycerine was found to be a much more powerful explosive than black powder, which is a physical mixture of potassium nitrate, sulfur, and charcoal.
– In black powder, potassium nitrate is the oxidizer, and sulfur and charcoal provide the fuel to be oxidized.
– The rate of a black powder explosion is limited by how fast oxygen from the grains of heated potassium nitrate can diffuse to the grains of sulfur and charcoal.
– A black powder explosion does its work by the rapid increase in pressure resulting from the reaction.
– The explosion must be confined, as in a cannon or a firecracker, to be effective.
– In nitroglycerine, the nitro groups are the oxidizer and the CH and CH2 groups are the fuel to be oxidized.
– This intimate association of fuel and oxidizer allows the explosion to proceed at a much faster rate, forming a shock wave that propagates through the explosive and initiates the reaction.
– The explosive shock wave can shatter rock or other substances without the need for confinement. Because of its unprecedented explosive power, nitroglycerine was called a high explosive.
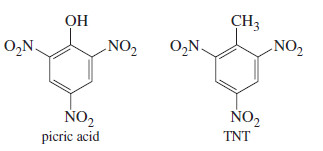
– Many other high explosives have been developed, including picric acid, TNT (trinitrotoluene), PETN (pentaerythritol tetranitrate), and RDX (research department explosive).
– Nitroglycerine and PETN are nitrate esters. Picric acid and TNT are nitrobenzene derivatives, not esters.
– Pure nitroglycerine is hazardous to make, use, and transport.
– Alfred Nobel’s family were experts at making and using nitroglycerine, yet his brother and several workers were killed by an explosion.
– In 1866, Nobel found that nitroglycerine soaks into diatomaceous earth to give a pasty mixture that can be molded into sticks that do not detonate so easily.
– He called the sticks dynamite and founded the firm Dynamit Nobel, which is still one of the world’s leading ammunition and explosives manufacturers.
– The Nobel prizes are funded from an endowment that originated with Nobel’s profits from the dynamite business.
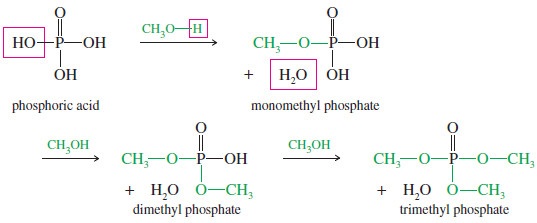
Phosphate Esters
– Alkyl phosphates are composed of 1 mole of phosphoric acid combined with 1, 2, or 3 moles of an alcohol.
– For example, methanol forms three phosphate esters.
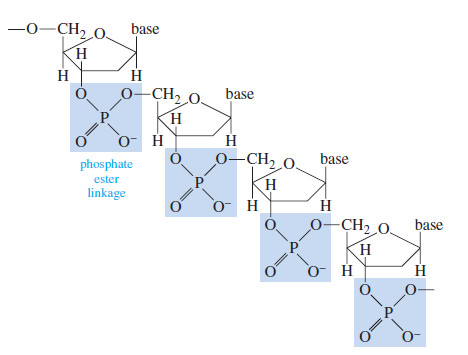
– Phosphate esters play a central role in biochemistry.
– The following Figure shows how phosphate ester linkages compose the backbone of the nucleic acids RNA (ribonucleic acid) and DNA (deoxyribonucleic acid).