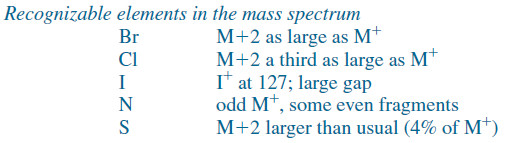Determination of the Molecular Formula by Mass Spectrometry
Determination of the Molecular Formula by Mass Spectrometry
– we can Determine the Molecular Formula by Mass Spectrometry and we will discuss :
(A) High-Resolution Mass Spectrometry
(B) Use of Heavier Isotope Peaks
(A) High-Resolution Mass Spectrometry
– Although mass spectra usually show the particle masses rounded to the nearest whole number, the masses are not integral.
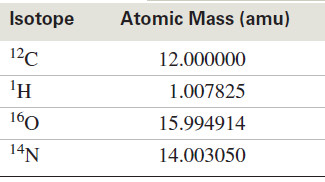
– The 12C nucleus is defined to have a mass of exactly 12 atomic mass units (amu), and all other nuclei have masses based on this standard.
– For example, a proton has a mass of about 1, but not exactly: Its mass is 1.007825 amu.
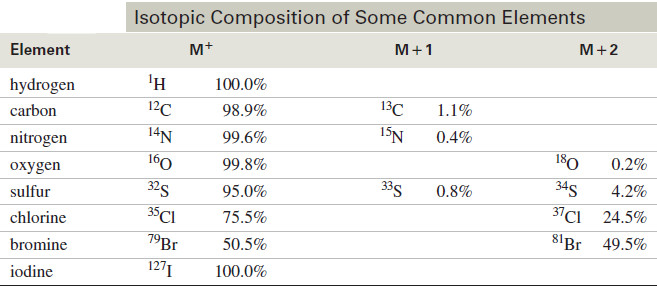
– The following table shows the atomic masses of the most common isotopes found in organic compounds.
– Determination of a molecular formula is possible using a high-resolution mass spectrometer (HRMS), one that uses extra stages of electrostatic or magnetic focusing to form a very precise beam and to detect particle masses to an accuracy of about 1 part in 20,000.
– A mass determined by several significant figures using an HRMS is called an exact mass.
– Although it is not really exact, it is much more accurate than the usual integral mass numbers.
– Comparing the exact mass with masses calculated by molecular formula makes it possible to identify the correct formula.
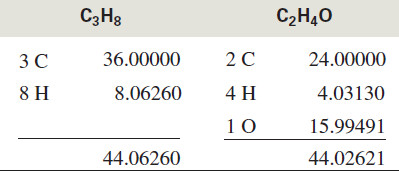
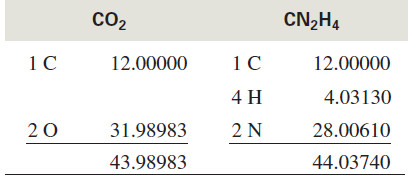
– Consider a molecular ion with a mass of 44.
– This approximate molecular weight might correspond to C3H8 (propane), C2H4O (acetaldehyde), CO2, or CN2H4. Each of these molecular formulas corresponds to a different exact mass:
– If the HRMS measured the exact mass of this ion as 44.029 mass units, we would conclude that the compound has a molecular formula of C2H4O because the mass corresponding to this formula is closest to the observed value.
– Published tables of exact masses are available for comparison with values obtained from the HRMS.
– Depending on the completeness of the tables, they may include sulfur, halogens, or other elements.
(B) Use of Heavier Isotope Peaks
– Whether or not a high-resolution mass spectrometer is available, molecular ion peaks often provide information about the molecular formula.
– Most elements do not consist of a single isotope but contain heavier isotopes in varying amounts.
– These heavier isotopes give rise to small peaks at higher mass numbers than the major M+ molecular ion peak.
– A peak that is one mass unit heavier than the M+ peak is called the M+1 peak; two units heavier, the M+2, and so on.
– The following Table gives the isotopic compositions of some common elements, showing how they contribute to M+1 and M+2 peaks.
– Ideally, we could use the isotopic compositions in the Table above to determine the entire molecular formula of a compound, by carefully measuring the abundances of the M+, M+1, and M+2 peaks.
– In practice, however, there are background peaks at every mass number.
– These background peaks are often similar in intensity to the M+1 peak, preventing an accurate measurement of the M+1 peak.
– High-resolution mass spectrometry is much more reliable.
Molecular ion peaks of Some elements
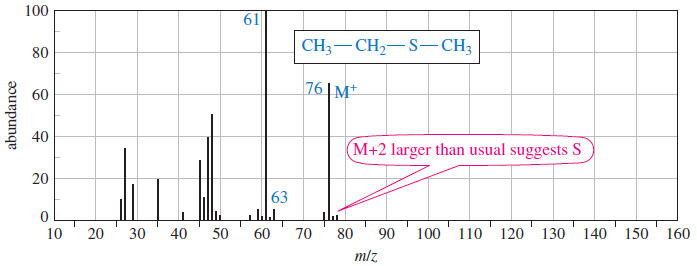
– Some elements (particularly S, Cl, Br, I, and N) are recognizable from molecular ion peaks, however, as the spectra shown next illustrate.
– A typical compound with no sulfur, chlorine, or bromine has a small M+1 peak and an even smaller (or no visible) M+2 peak.
– If a compound contains sulfur, the M+2 peak is larger than the M+1 peak: about 4% of the M+ peak.
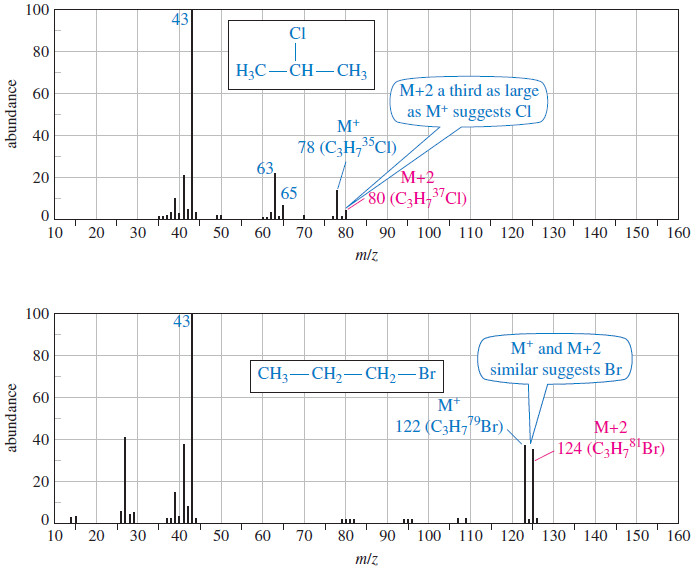
– If chlorine is present, the M+2 peak (containing 37Cl) is about a third as large as the M+ peak (containing 35Cl).
– And If bromine is present, the M+ and M+2, ions have about equal abundances; the molecular ion appears as a doublet separated by two mass units, with one mass corresponding to 79Br and one to 81 Br.
– Iodine is recognized by the presence of the iodonium ion, I+ at m/z 127.
– This clue is combined with a characteristic 127-unit gap in the spectrum corresponding to the loss of the iodine radical.
– Nitrogen (or an odd number of nitrogen atoms) gives an odd molecular weight and usually gives some major even-numbered fragments.
– Stable compounds containing only carbon, hydrogen, and oxygen have even molecular weights, and most of their major fragments are odd-numbered.
– The following spectra show compounds containing sulfur, chlorine, and bromine.