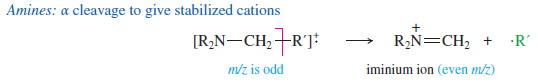Fragmentation Patterns in Mass Spectrometry
– In this topic, we will discuss the Fragmentation Patterns in Mass Spectrometry.
Fragmentation Patterns in Mass Spectrometry
– In addition to the molecular formula, the mass spectrum provides structural information.
– An electron with a typical energy of 70 eV (6740 kJ mol or 1610 kcal mol) has far more energy than needed to ionize a molecule.
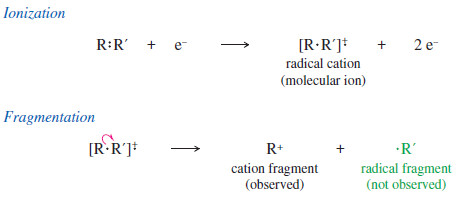
– The impact forms the radical cation, and it often breaks a bond to give a cation and a radical.
– The resulting cation is observed by the mass spectrometer, but the uncharged radical is not accelerated or detected.
– We can infer the mass of the uncharged radical from the amount of mass lost from the molecular ion to give the observed cation fragment.
– This bond breaking does not occur randomly; it tends to form the most stable fragments.
– By knowing what stable fragments result from different kinds of compounds, we can recognize structural features and use the mass spectrum to confirm a proposed structure
Mass Spectra of Alkanes
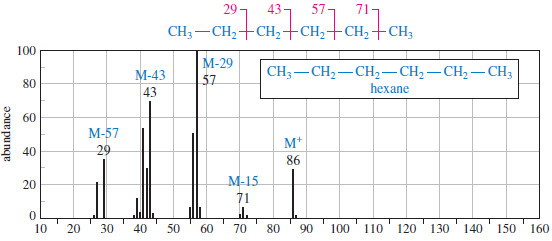
– The mass spectrum of hexane shows several characteristics typical of straight-chain alkanes.
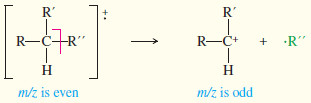
– Like other compounds not containing nitrogen, the molecular ion (M+) has an even-numbered mass, and most of the fragments are odd-numbered.
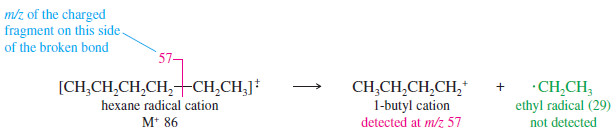
– The base peak (m>z 57) corresponds to loss of an ethyl group, giving an ethyl radical and a butyl cation.
– The neutral ethyl radical is not detected, because it is not charged and is not accelerated or deflected.
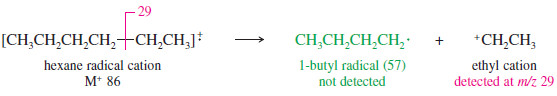
– A similar fragmentation gives an ethyl cation and a butyl radical. In this case, the ethyl fragment (m/z 29) is detected.
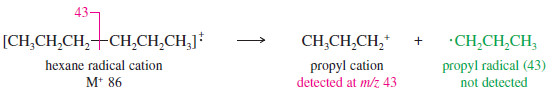
– Symmetric cleavage of hexane gives a propyl cation and a propyl radical.
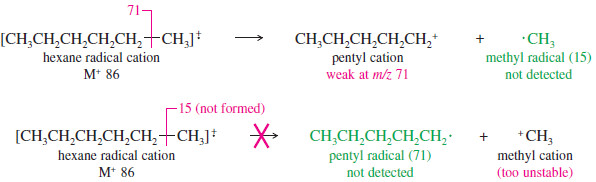
– Cleavage to give a pentyl cation (m/z 71) and a methyl radical is weak because the methyl radical is less stable than a substituted radical.
– Cleavage to give a methyl cation (m/z 15) and a pentyl radical is not visible because the methyl cation is less stable than a substituted cation.
– The stability of the cation is apparently more important than the stability of the radical, since a weak peak appears corresponding to loss of a methyl radical, but we see no cleavage to give a methyl cation.
– Cation and radical stabilities help to explain the mass spectra of branched alkanes as well.
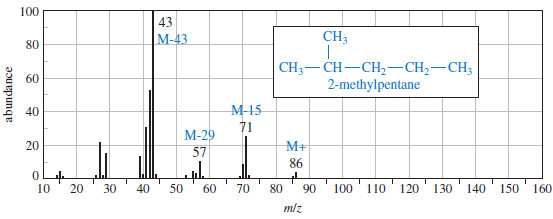
– The following Figure shows the mass spectrum of 2-methylpentane.
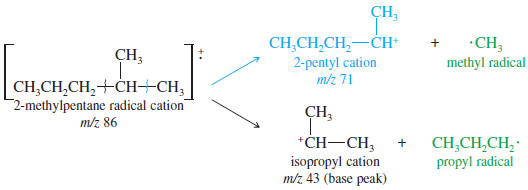
– Fragmentation of a branched alkane commonly occurs at a branch carbon atom to give the most highly substituted cation and radical.
– Fragmentation of 2-methylpentane at the branched carbon atom can give a secondary carbocation in either of two ways:
– Both fragmentations give secondary cations, but the second gives a primary radical instead of a methyl radical.
– Therefore, the second fragmentation accounts for the base (largest) peak, while the first accounts for another large peak at m/z 71.
– Other fragmentations (to give primary cations) account for the weaker peaks.
Fragmentation Giving Resonance-Stabilized Cations
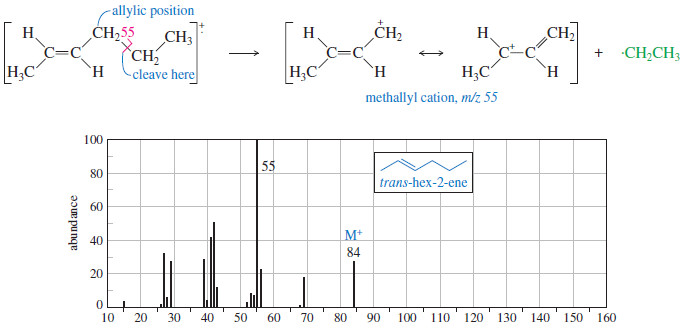
– Fragmentation in the mass spectrometer gives resonance-stabilized cations whenever possible.
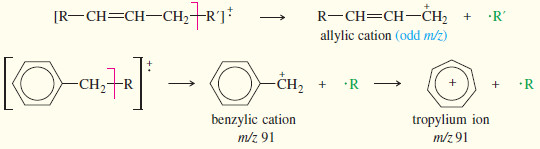
– The most common fragmentation of alkenes is cleavage of an allylic bond to give a resonance-stabilized allylic cation.
– The following Figure shows how the radical cation of trans-hex-2-ene undergoes allylic cleavage to give the resonance-stabilized cation responsible for the base peak at m/z 55.
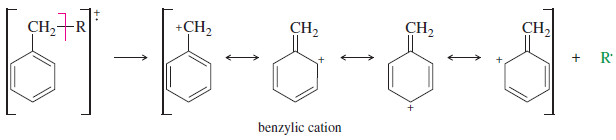
– Compounds containing aromatic rings tend to fragment at the carbon (called a benzylic carbon) next to the aromatic ring. Such a cleavage forms a resonance-stabilized benzylic cation.
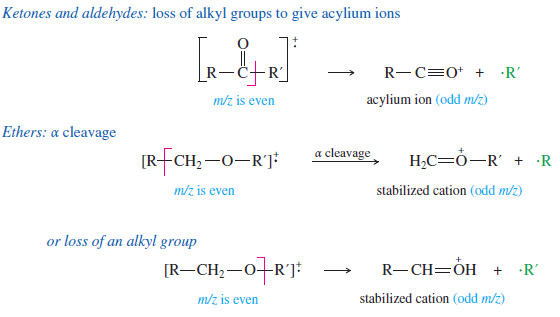
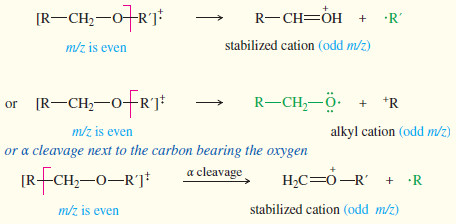
– Ethers, amines, and carbonyl compounds can also fragment to give resonancestabilized cations.
– The oxygen and nitrogen atoms in these compounds have nonbonding electrons that can stabilize the positive charge of a cation through resonance forms with octets on all the atoms.
– Common fragmentations often cleave the bond next to the carbon atom bearing the oxygen or nitrogen.
– We will see examples of these favorable fragmentations in later chapters covering the chemistry of these functional groups.
Fragmentation Splitting Out a Small Molecule; Mass Spectra of Alcohols
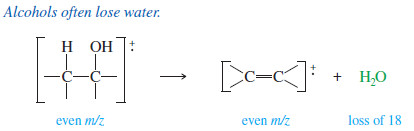
– Mass spectral peaks are often seen corresponding to loss of small, stable molecules.
– Loss of a small molecule is usually indicated by a fragment peak with an even mass number, corresponding to loss of an even mass number.
– A radical cation may lose water (mass 18), CO (28), CO2 (44), and even ethene (28) or other alkenes.
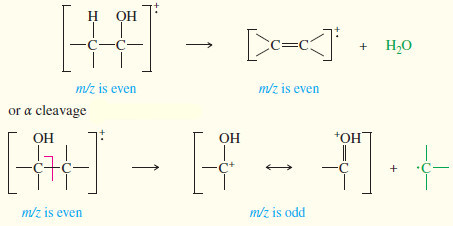
– The most common example is the loss of water from alcohols, which occurs so readily that the molecular ion is often weak or absent.
– The peak corresponding to loss of water (the M–18 peak) is usually strong, however.
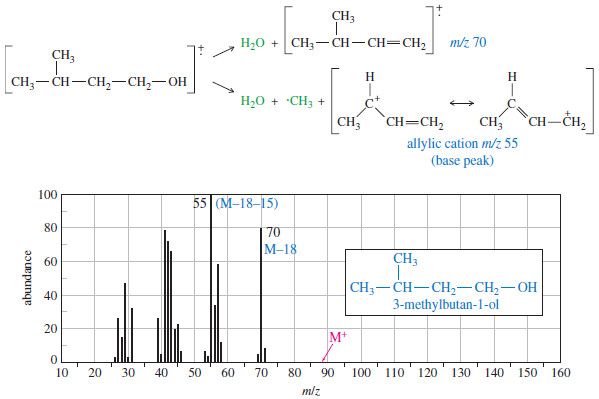
– The mass spectrum of 3-methylbutan-1-ol shows a favorable loss of water. (see figure below)
– The even-numbered peak at m/z 70 that appears to be the molecular ion is actually the intense M–18 peak. The molecular ion (m/z 88) is not observed because it loses water very readily.
– The base peak at m/z 55 corresponds to loss of water and a methyl group.
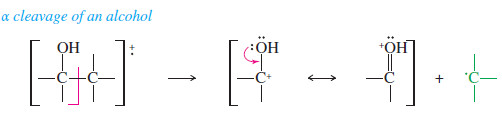
– In addition to losing water, alcohols commonly fragment next to the carbinol carbon atom to give a resonance stabilized carbocation.
– This fragmentation is called an alpha cleavage because it breaks the bond next to the carbon bearing the hydroxyl group.
SUMMARY: Common Fragmentation Patterns
– This summary is provided for rapid reference to the common fragmentation patterns of simple functional groups.
(1) Alkanes: cleavage to give the most stable carbocations
(2) Alcohols: loss of water
(3) Alkenes and aromatics: cleavage to give allylic and benzylic carbocations
(4) Amines: α cleavage next to the carbon bearing the nitrogen to give stabilized cations
(5) Ethers: loss of an alkyl group
(6) Ketones and aldehydes: loss of alkyl groups next to the carbon bearing the oxygen to give acylium ions.
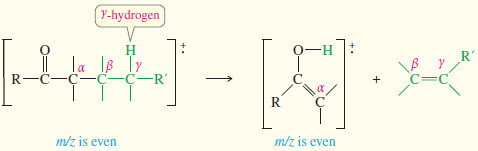
The McLafferty rearrangement splits out alkenes