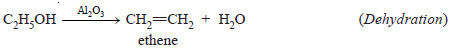Characteristics of Catalytic Reactions
– In this topic, we will discuss general Characteristics of Catalytic Reactions and Promoters.
General Characteristics of Catalytic Reactions
– Although there are different types of catalytic reactions, the following features or characteristics are common to most of them.
(1) A catalyst remains unchanged in mass and chemical composition at the end of the reaction
– Qualitative and quantitative analysis show that a catalyst undergoes no change in mass of chemical nature. However, it may undergo a physical change.
– Thus granular manganese dioxide (MnO2) used as a catalyst in the thermal decomposing of potassium chlorate is left as a fine powder at the end to the reaction.
(2) A small quantity of catalyst is generally needed to produce almost unlimited reaction
– Sometimes a trace of a metal catalyst is required to affect very large amounts of reactants.
– For example, one ten millionth of its mass of finely divided platinum is all that is needed to catalyse the decomposition of hydrogen peroxide.
– On the other hand, there are catalysts which need to be present in relatively large amount to be effective.
– Thus in Friedel-Crafts reaction,
anhydrous aluminium chloride functions as a catalyst effectively when present to the extent of 30 per cent of the mass of benzene.
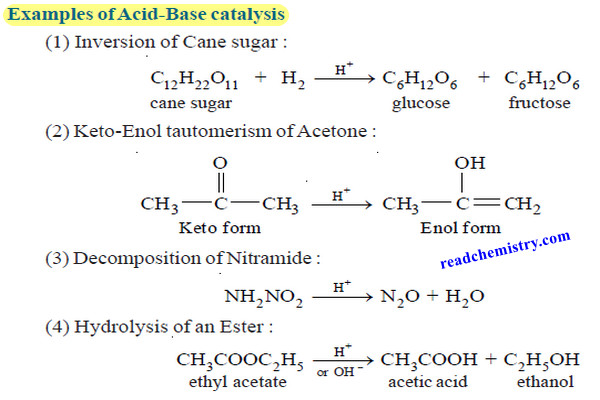
– For the acid and alkaline hydrolysis of an ester,
the rate of reaction is proportional to the concentration of the catalyst (H+ or OH– ).
(3) A catalyst is more effective when finely divided
– In heterogeneous catalysis, the solid catalyst is more effective when in a state of fine subdivision than it is used in bulk.
– Thus a lump of platinum will have much less catalytic activity than colloidal or platinised asbestos.
– Finely divided nickel is a better catalyst than lumps of solid nickel.
(4) A catalyst is specific in its action
– While a particular catalyst works for one reaction, it will not necessarily work for another reaction.
– Different catalysts, moreover, can bring about completely different reactions for the same substance.
– For example, ethanol (C2H5OH) gives ethene (C2H4) when passed over hot aluminium oxide,
but with hot copper it gives ethanal (CH3CHO).
(5) A catalyst cannot, in general, initiate a reaction
– In most cases a catalyst speeds up a reaction already in progress and does not initiate (or start) the reaction.
– But there are certain reactions where the reactants do not combine for very long period (perhaps years).
– For example, a mixture of hydrogen and oxygen, which remains unchanged almost indefinitely at room temperature, can be brought to reaction by the catalyst platinum black in a few seconds.
– Thus it is now considered that the catalyst can initiate a reaction.
– According to this view, the reacting molecules (in the absence of catalyst) do not possess minimum kinetic energies for successful collisions.
– The molecules rebound from collision without reacting at all.
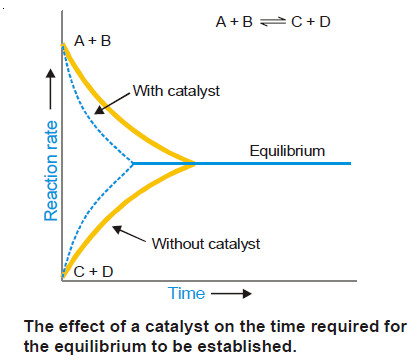
(6) A catalyst does not affect the final position of equilibrium, although it shortens the time required to establish the equilibrium
– It implies that in a reversible reaction the catalyst accelerates the forward and the reverse reactions equally.
– Thus the ratio of the rates of two opposing reactions i.e., the equilibrium constant, remains unchanged.
– The effect of a catalyst on the time required for equilibrium to be established for the reaction is illustrated in Fig below
– To start with the concentrations of A and B are at the maximum and hence the rate of forward reaction is maximum.
– As the time passes the rate of the reaction decreases till the equilibrium is established. For the reverse reaction the initial concentrations of C and D are zero and the rate of reaction is lowest.
– At the time passes, the rate of reaction increases till the equilibrium is established.
– Similar curves of the rates of reactions with the catalyst show that the rates of the forward reaction and the reverse reaction are altered equally but the equilibrium is established in a much shorter time.
– For example, in the Haber Process for ammonia,
the reaction is very slow.
– In the presence of the catalyst, the equilibrium is reached much sooner but the percentage yield remains unchanged.
– The iron catalyst shortens the time to attain equilibrium but cannot alter the percentage yield.
– Energy considerations also show that the final state of equilibrium cannot be changed by the catalyst.
– Suppose the catalyst accelerates the forward reaction more than the reverse reaction.
– This will shift the equilibrium point, which cannot happen without the supply of energy to the system.
– But a catalyst unchanged in mass and composition at the end of the reaction, cannot supply the required energy.
(7) Change of temperature alters the rate of a catalytic reaction as it would do for the same reaction without a catalyst
– We have already studied the effect of temperature change on reversible reactions under Le Chatelier principle.
– Some catalysts are, however, physically altered by a rise in temperature and hence their catalytic activity may be decreased.
– This is particularly true with colloidal solutions like that of platinum, since a rise in the temperature may cause their coagulation.
– In such a case the rate of reaction increases up to a certain point and then gradually decreases.
– The rate of reaction is maximum at a particular temperature called the optimum temperature.
Promoters
– The activity of a catalyst can often be increased by addition of a small quantity of a second material.
– This second substance is either not a catalyst itself for the reaction or it may be a feeble catalyst.
– A substance which, though itself not a catalyst, promotes the activity of a catalyst is called a promoter.
Example of Promoters
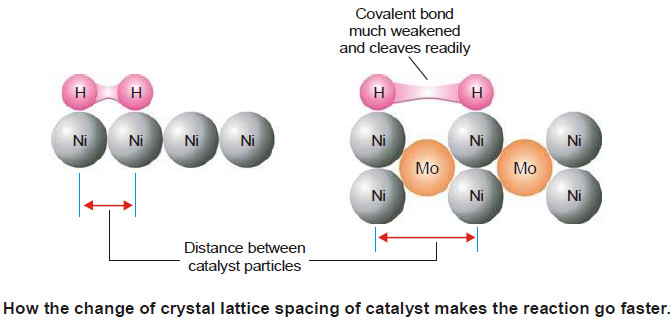
– Molybdenum (Mo) or aluminium oxide (Al2O3) promotes the activity of iron catalyst in the Haber synthesis for the manufacture of ammonia.
– In some reactions, mixtures of catalysts are used to obtain the maximum catalytic efficiency.
– For example, in the synthesis of methanol (CH3OH) from carbon monoxide and hydrogen, a mixture of zinc and chromium oxide is used as a catalyst.
Explanation of Promotion Action
The theory of promotion of a catalyst is not clearly understood. Presumably :
(1) Change of Lattice Spacing.
– The lattice spacing of the catalyst is changed thus enhancing the spaces between the catalyst particles.
– The absorbed molecules of the reactant (say H2) are further weakened and cleaved. This makes are reaction go faster.
(2) Increase of Peaks and Cracks.
– The presence of the promoter increases the peaks and cracks on the catalyst surface.
– This increases the concentration of the reactant molecules and hence the rate of reaction.
– The phenomenon of promotion is a common feature of heterogeneous catalysis.