Lyophilic and Lyophobic sols : Defination, Properties, Comparison
– In this topic, we will discuss The Lyophilic and Lyophobic sols : Defination, Characteristics, and Comparison.
Defination of Lyophilic and Lyophobic sols
– Sols are colloidal systems in which a solid is dispersed in a liquid.
– These can be subdivided into two classes :
- Lyophilic sols (solvent-loving)
- Lyophobic sols (solvent-hating)
– Lyophilic sols are those in which the dispersed phase exhibits a definite affinity for the medium or the solvent.
– The examples of lyophilic sols are dispersions of starch, gum, and protein in water.
– Lyophobic sols are those in which the dispersed phase has no attraction for the medium or the solvent.
– The examples of lyophobic sols are dispersion of gold, iron (III) hydroxide and sulphur in water.
– The affinity or attraction of the sol particles for the medium, in a lyophilic sol, is due to hydrogen bonding with water.
– If the dispersed phase is a protein (as in egg) hydrogen bonding takes place between water molecules and the amino groups ( –NH–, –NH2) of the protein molecule.
– In a dispersion of starch in water, hydrogen bonding occurs between water molecules and the – OH groups of the starch molecule.
– There are no similar forces of attraction when sulphur or gold is dispersed in water.
Properties of Lyophilic and Lyophobic sols
– Some features of lyophilic and lyophobic sols are listed below as follow:
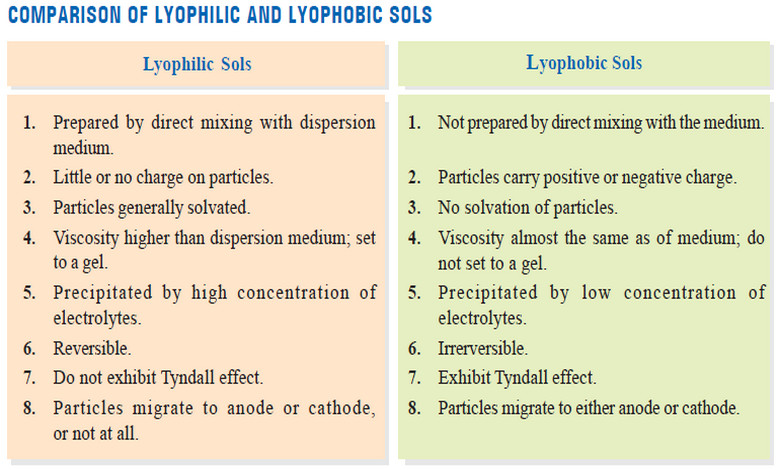
(1) Ease of preparation
– Lyophilic sols can be obtained straightaway by mixing the material (starch, protein) with a suitable solvent.
– The giant molecules of the material are of colloidal size and these at once pass into the colloidal form on account of interaction with the solvent.
– Lyophobic sols are not obtained by simply mixing the solid material with the solvent.
(2) Charge on particles
– Particles of a hydrophilic sol may have little or no charge at all.
– Particles of a hydrophobic sol carry positive or negative charge which gives them stability.
(3) Solvation
– Hydrophilic sol particles are generally solvated.
– That is, they are surrounded by an adsorbed layer of the dispersion medium which does not permit them to come together and coagulate.
– Hydration of gelatin is an example.
– There is no solvation of the hydrophobic sol particles for want of interaction with the medium.
(4) Viscosity
– Lyophilic sols are viscous as the particle size increases due to solvation, and the proportion of free medium decreases.
– Warm solutions of the dispersed phase on cooling set to a gel e.g., preparation of table jelly.
– Viscosity of hydrophobic sol is almost the same as of the dispersion medium itself.
(5) Precipitation
– Lyophilic sols are precipitated (or coagulated) only by high concentration of the electrolytes when the sol particles are dissolved.
– Lyophobic sols are precipitated even by low concentration of electrolytes, the protective layer
being absent.
(6) Reversibility
– The dispersed phase of lyophilic sols when separated by coagulation or by evaporation of the
medium, can be reconverted into the colloidal form just on mixing with the dispersion medium.
– Therefore this type of sols are designated as Reversible sols.
– On the other hand, the lyophobic sols once precipitated cannot be reformed merely by mixing with dispersion medium. These are, therefore, called Irreversible sols.
(7) Tyndall effect
– On account of relatively small particle size, lyophilic sols do not scatter light and show no Tyndall effect.
– Lyophobic sol particles are large enough to exhibit tyndall effect.
(8) Migration in electronic field
– Lyophilic sol particles (proteins) migrate to anode or cathode, or not at all, when placed in electric field.
– Lyophobic sol particles move either to anode or cathode, according as they carry negative or positive charge.
Comparison of Lyophilic and Lyophobic sols
Preparation of Lyophilic and Lyophobic sols
– Lyophilic sols may be prepared by simply warming the solid with the liquid dispersion medium e.g., starch with water.
– On the other hand, lyophobic sols have to be prepared by special methods
– These methods fall into two categories :
(a) Dispersion Methods in which larger macro-sized particles are broken down to colloidal size.
(b) Aggregation Methods in which colloidal size particles are built up by aggregating single ions or molecules.