Complex Splitting in ¹H NMR Spectra
– In the last topic we talk about Spin-Spin Splitting in ¹H NMR Spectra, but In this topic, we will discuss The Complex Splitting.
Complex Splitting in ¹H NMR Spectra
– There are many cases of complex splitting, where signals are split by adjacent protons of more than one type, with different coupling constants.
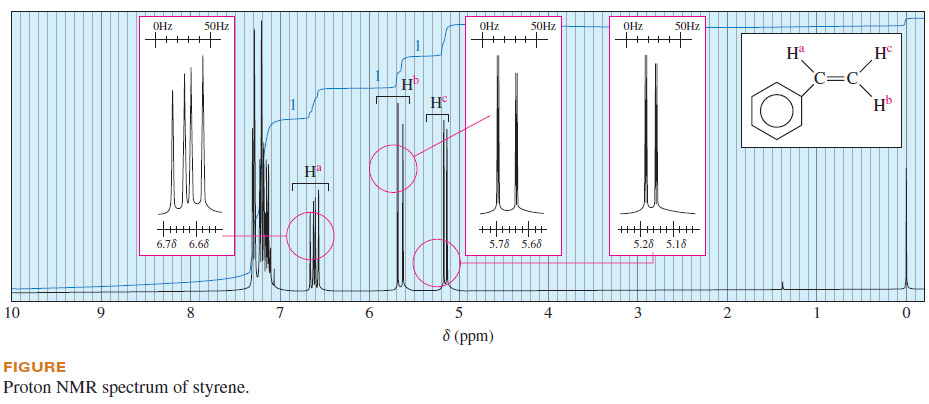
– The proton NMR spectrum of styrene (Figure) includes several absorptions that show the results of complex splitting.
– Consider the vinyl proton Ha, adjacent to the phenyl ring of styrene.
– The chemical shift of Ha is δ 6.6 because it is deshielded by both the vinyl group and the aromatic ring.
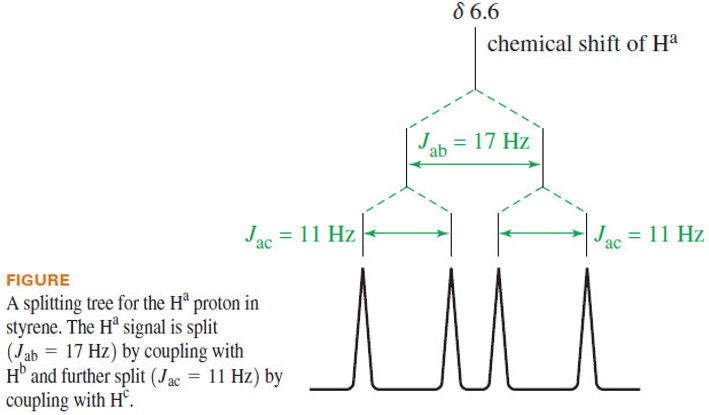
– Ha is coupled to Hb with a typical trans coupling constant, Jab = 17 Hz.
– It is also coupled to proton with The signal is therefore split into a doublet of spacing 17 Hz, and each of those peaks is further split into a doublet of spacing 11 Hz, for a total of four peaks.
– This complex splitting, called a doublet of doublets, can be analyzed by a diagram called a splitting tree, shown in the following Figure
– Proton Hb is farther from the deshielding influence of the phenyl group, giving rise to the multiplet centered at δ 5.65 in the styrene NMR spectrum.
– Hb is also split by two nonequivalent protons: It is split by Ha with a trans coupling constant Jac = 17 Hz and further split by Hc with a geminal coupling constant Jbc = 1.4 Hz.
– The splitting tree for showing a doublet of narrow doublets, is shown in the following Figure.
– Sometimes a signal is split by two or more different kinds of protons with similar coupling constants.
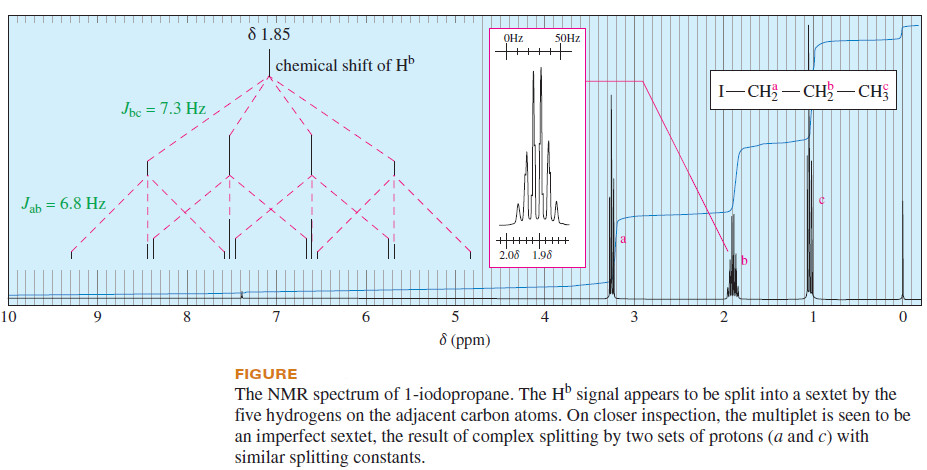
– Consider 1-iodopropane (the following Figure), where the b protons on the middle carbon atom are split by two types of protons: the methyl protons (Hc) and the CH2I protons (Ha).
– The coupling constants for these two interactions are similar: Jab = 6.8 Hz, and Jbc = 7.3 Hz.
– The spectrum shows the Hb signal as a sextet, almost as though there were five equivalent protons coupled with Hb.
– The trace in the insert box, enlarged and offset, shows that the pattern is not a perfect sextet.
– The analysis of the splitting pattern serves as a reminder that the N+1 rule gives a perfect multiplet only when the signal is split by N protons with exactly the same coupling constant.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.