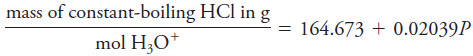Reagents for Neutralization Titrations
– In this subject, we will discuss the Reagents for Neutralization Titrations.
Reagents for Neutralization Titrations
– we noted before that strong acids and strong bases produce the largest change in pH at the equivalence point.
– For this reason, standard solutions for neutralization titrations are always prepared from these reagents.
Preparation of Standard Acid Solutions
– Hydrochloric acid solutions are widely used as standard solutions for titrating bases.
– Dilute solutions of HCl are stable indefinitely, and many chloride salts are soluble in aqueous solution.
– Solutions of 0.1 M HCl can be boiled for as long as an hour without loss of acid, provided that the water lost by evaporation is periodically replaced; 0.5 M solutions can be boiled for at least ten minutes without significant loss.
– Solutions of perchloric acid and sulfuric acid are also stable and are useful for titrations where chloride ion interferes by forming precipitates.
– Standard solutions of nitric acid are seldom used because of their oxidizing properties.
– To obtain most standard acid solutions, a solution of an approximate concentration is first prepared by diluting the concentrated reagent.
– The diluted acid solution is then standardized against a primary-standard base.
– Occasionally, the composition of the concentrated acid is obtained by careful density measurements.
– A weighed quantity of the concentrated acid is then diluted to a known volume.
– Most chemistry and chemical engineering handbooks contain tables relating reagents density to composition.
– A stock solution with a known hydrochloric acid concentration can also be prepared by diluting a quantity of the concentrated reagents with an equal volume of water followed by distillation.
– Under controlled conditions, the final quarter of the distillate, known as constant-boiling HCl, has a constant and known composition.
– The acid content of constant-boiling HCl depends only on atmospheric pressure.
– For a pressure P between 670 and 780 torr, the mass in air of the distillate that contains exactly one mole of H3O+ is:
– Standard solutions are prepared by diluting weighed quantities of this acid to accurately known volumes.
The Standardization of Acids
– Sodium carbonate is the one of most frequently used reagents for standardizing acids.
– Several other reagents are also used.
Sodium Carbonate
– Primary-standard-grade sodium carbonate is available commercially or can be prepared by heating purified sodium hydrogen carbonate between 270 to 300°C for 1 hr.
2HCO3 (s) → Na2CO3 + H2O + CO2 (g)
– An accurately determined mass of the primary-standard material is then taken to standardize the acid.
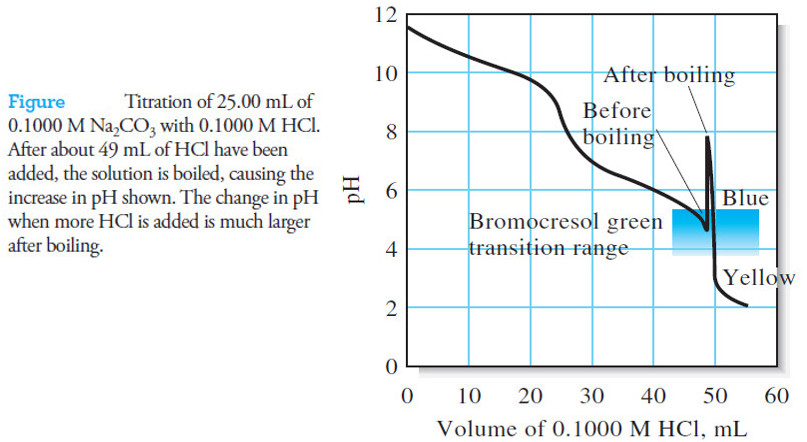
– As shown in the following Figure , there are two end points in the titration of sodium carbonate.
– The first, corresponding to the conversion of carbonate to hydrogen carbonate, occurs at about pH 8.3; the second, involving the formation of carbonic acid and carbon dioxide, appears at about pH 3.8.
– The second end point is always used for standardization because the change in pH is greater than that at the first.
– An even sharper end point can be achieved by boiling the solution briefly to eliminate the reaction product, carbonic acid and carbon dioxide.
– The sample is titrated to the first appearance of the acid color of the indicator (such as bromocresol green or methyl orange).
– At this point, the solution contains a large amount of dissolved carbon dioxide and small amounts of carbonic acid and unreacted hydrogen carbonate.
– Boiling effectively destroys this buffer by eliminating the carbonic acid:
H2CO3 (aq) → CO2 (g) + H2O(l)
– The solution then becomes alkaline again due to the residual hydrogen carbonate ion.
– The titration is completed after the solution has cooled, resulting in a substantially larger decrease in pH during the final additions of acid.
– A more abrupt color change (see Figure above) is the result.
– Alternatively, an amount of acid slightly in excess of that needed to convert the sodium carbonate to carbonic acid can be introduced.
– The solution is boiled as before to remove carbon dioxide and cooled.
– The excess acid is then back-titrated with a dilute solution of standard base.
– Any indicator suitable for a strong acid/strong base titration is satisfactory.
– An independent titration is used to establish the volume ratio of acid to base.
Other Primary Standards for Acids
– Tris-(hydroxymethyl)aminomethane, (HOCH2)3CNH2, known also as TRIS or THAM, is available in primary-standard purity from commercial sources.
– The main advantage of TRIS is its much greater mass per mole of protons consumed (121.1 g/mol) than sodium carbonate (53.0 g/mol).
– The reaction of TRIS with acids is
(HOCH2)3CNH2 + H3O+ ↔ (HOCH2)3CNH3+ + H2O
– Sodium tetraborate decahydrate and mercury(II) oxide have also been recommended as primary standards.
– The reaction of an acid with the tetraborate is
B4O7– + 2H3O+ + 3H2O → 4H3BO3
Preparation of Standard Solutions of Base
– Sodium hydroxide is the most common base for preparing standard solutions (reagents), although potassium hydroxide and barium hydroxide are also used.
– These bases cannot be obtained in primary standard purity, and so, all must be standardized after they are prepared.
The Effect of Carbon Dioxide on Standard Base Solutions
– In solution as well as in the solid state, the hydroxides of sodium, potassium, and barium react rapidly with atmospheric carbon dioxide to produce the corresponding carbonate:
CO2 (g) + 2OH– → CO32- + H2O
– Although production of each carbonate ion consumes two hydroxide ions, the uptake of carbon dioxide by a solution of base does not necessarily alter its combining capacity for hydronium ions.
– Thus, at the end point of a titration that requires an acid-range indicator (such as bromocresol green), each carbonate ion produced from sodium or potassium hydroxide will have reacted with two hydronium ions of the acid (see Figure above):
CO3 2- + 2H3O+ → H2CO3 + 2H2O
– Because the amount of hydronium ion consumed by this reaction is identical to the amount of hydroxide lost during formation of the carbonate ion, no error results from the reaction of the hydroxide with CO2.
– Unfortunately, most applications of standard base require an indicator with a basic transition range (phenolphthalein, for example).
– In this case, carbonate ion has reacted with only one hydronium ion when the color change of the indicator is observed:
CO32- + H3O+ → HCO3– + H2O
– The effective concentration of the base is thus diminished by absorption of carbon dioxide (a systematic error)
– The solid reagents used to prepare standard solutions of base are always contaminated by significant amounts of carbonate ion.
– The presence of this contaminant does not cause a carbonate error provided the same indicator is used for both standardization and analysis.
– Carbonate does, however, decrease the sharpness of end points.
– For this reason, carbonate ion is usually removed before a solution of a base is standardized.
– The best method for preparing carbonate-free sodium hydroxide solutions takes advantage of the very low solubility of sodium carbonate in concentrated solutions of the base.
– An approximately 50% aqueous solution of sodium hydroxide is prepared or purchased from commercial sources.
– The solid sodium carbonate is allowed to settle to give a clear liquid that is decanted and diluted to give the desired concentration.
– Alternatively, the solid can be removed by vacuum filtration.
– Water that is used to prepare carbonate-free solutions of base must also be free of carbon dioxide.
– Distilled water, which is sometimes supersaturated with carbon dioxide, should be boiled briefly to eliminate CO2.
– The water is then allowed to cool to room temperature before base is introduced because hot alkali solutions rapidly absorb carbon dioxide.
– Deionized water usually does not contain significant amounts of carbon dioxide.
– A tightly capped low-density polyethylene bottle usually provides adequate shortterm protection against the uptake of atmospheric carbon dioxide.
– Before capping, the bottle is squeezed to minimize the interior air space.
– Care should also be taken to keep the bottle closed except during the brief periods when the contents are being transferred to a buret.
– Over time, sodium hydroxide solutions cause polyethylene bottles to become brittle.
– The concentration of a sodium hydroxide solution will decrease slowly (0.1% to 0.3% per week) if the base is stored in glass bottles.
– The loss in strength is caused by the reaction of the base with the glass to form sodium silicates.
– For this reason, standard solutions of base should not be stored for extended periods (longer than 1 or 2 weeks) in glass containers.
– In addition, bases should never be kept in glass-stoppered containers because the reaction between the base and the stopper may cause the latter to “freeze” after a brief period.
– Finally, to avoid the same type of freezing, burets with glass stopcocks should be promptly drained and thoroughly rinsed with water after use with standard base solutions.
– Most modern burets are equipped with Teflon stopcocks and thus do not have this problem.
The Standardization of Bases
– Several excellent primary standards are available for standardizing bases.
– Most are weak organic acids that require the use of an indicator with a basic transition range.
Potassium Hydrogen Phthalate
– Potassium hydrogen phthalate, KHC8H4O4 is a nearly ideal primary standard.
– It is a nonhygroscopic crystalline solid with a relatively large molar mass (204.2 g/mol).
– For most purposes, the commercial analytical-grade salt can be used without further purification.
– For the most exacting work, potassium hydrogen phthalate (KHP) of certified purity is available from the National Institute of Standards and Technology.
Other Primary Standards for Bases
– Benzoic acid can be obtained in primary-standard purity and used for the standardization of bases.
– Benzoic acid has limited solubility in water, so it is usually dissolved in ethanol prior to dilution with water and titration.
– A blank should always be carried through this standardization because commercial alcohol is sometimes slightly acidic.
– Potassium hydrogen iodate, KH(IO3)2, is an excellent primary standard with a high molar mass per mole of protons.
– It is also a strong acid that can be titrated using virtually any indicator with a transition range between pH 4 and 10.
Reference
- Modern analytical chemistry / David Harvey / The McGraw-Hill Companies, Inc./ , 2000 . USA
- Dean’s Analytical Chemistry Handbook / Pradyot Patnaik / The McGraw-Hill Companies, 2nd Editionm, 2004 .USA
- Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition) , 2014 . USA