Stereochemical Nonequivalence of Protons in NMR Spectroscopy
– In the this topic we talk about Stereochemical Nonequivalence of Protons in NMR Spectroscopy.
Stereochemical Nonequivalence of Protons
– Stereochemical differences often result in different chemical shifts for protons on the same carbon atom.
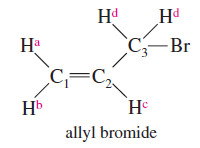
– For example, the two protons on C1 of allyl bromide (3-bromopropene) are not equivalent.
– Ha is cis to the -CH2Br group, and Hb is trans.
– Ha absorbs at δ 5.3; Hb absorbs at δ 5.1.
– There are four different (by NMR) types of protons in allyl bromide, as shown in the structure at right in the margin.
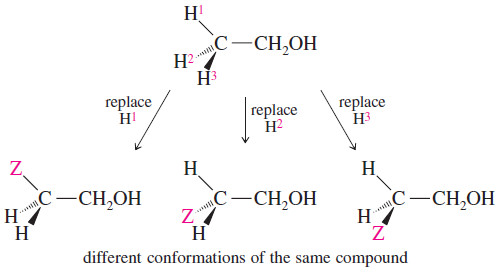
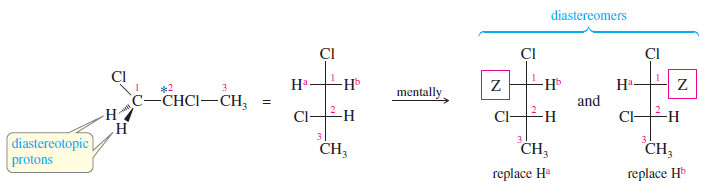
– To determine whether similar-appearing protons are equivalent, mentally substitute another atom for each of the protons in question.
– If the same product is formed by imaginary replacement of either of two protons, those protons are chemically equivalent.
– For example, replacing any of the three methyl protons in ethanol by an imaginary Z atom gives the same compound.
– These three hydrogens are chemically equivalent, and they will all appear at the same chemical shift.
– Freely rotating CH3 groups always have chemically equivalent protons.
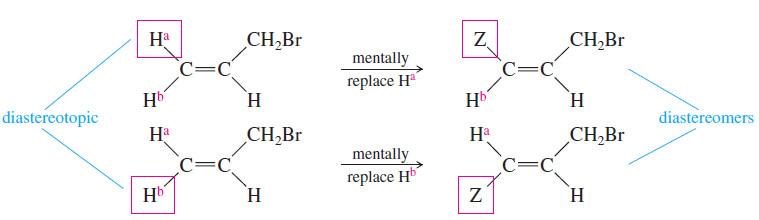
– When this imaginary replacement test is applied to the protons on C1 of allyl bromide, the imaginary products are different.
– Replacing the cis hydrogen gives the cis diastereomer, and replacing the trans hydrogen gives the trans diastereomer.
– Because the two imaginary products are diastereomers, these protons on C1 are called diastereotopic protons.
– Diastereotopic protons appear in the NMR at different chemical shifts, and they can split each other.
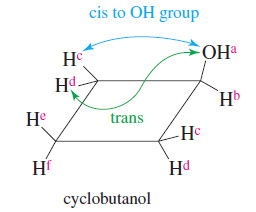
– Cyclobutanol shows these stereochemical relationships in a cyclic system.
– The hydroxyl proton Ha is clearly unique; it absorbs between δ 3 and δ 5, depending on the solvent and concentration.
– Hb is also unique, absorbing between δ 3 and δ 4.
– Protons and He are Hf diastereotopic (and absorb at different fields) because He is cis to the hydroxyl group, while Hf is trans.
– To distinguish among the other four protons, notice that cyclobutanol has an internal mirror
plane of symmetry.
– Protons Hc are cis to the hydroxyl group, while protons Hd are trans. Therefore, protons Hc are diastereotopic to protons Hd and the two sets of protons absorb at different magnetic fields and are capable of splitting each other.
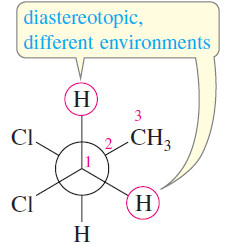
– Diastereomerism also occurs in saturated, acyclic compounds; for example, 1,2-dichloropropane is a simple compound that contains diastereotopic protons.
– The two protons on the -CH2Cl group are diastereotopic; their imaginary replacement gives diastereomers.
– The most stable conformation of 1,2-dichloropropane (in the margin) shows that the diastereotopic protons on C1 exist in different chemical environments.
– They experience different magnetic fields and are nonequivalent by NMR.
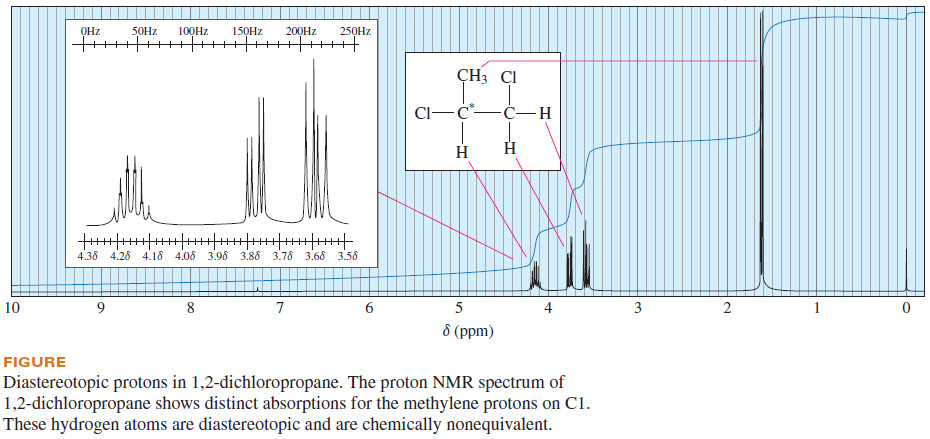
– The NMR spectrum of 1,2-dichloropropane is shown in The following Figure.
– The methyl protons appear as a doublet at δ 1.6, and the single proton on C2 appears as a complex multiplet at δ 4.15 .
– The two C1 protons on appear as distinct absorptions at δ 3.6 and δ 3.77 .They are split by the proton on C2 and they also split each other.
– The presence of an asymmetric carbon atom adjacent to the CH2Cl group gives rise to the different chemical environments of these diastereotopic protons.
– When a molecule contains an asymmetric carbon atom, the protons on any methylene group are usually diastereotopic.
– They may or may not be resolved in the NMR, however, depending on the differences in their environments.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.