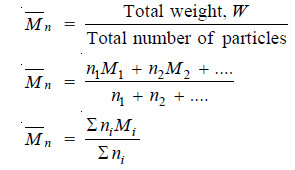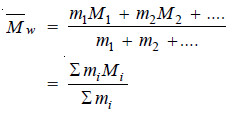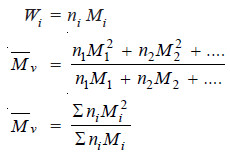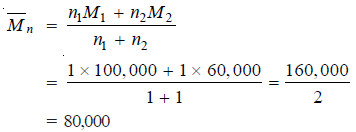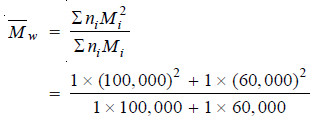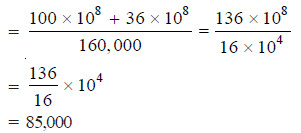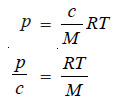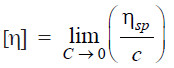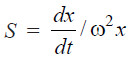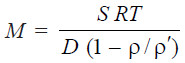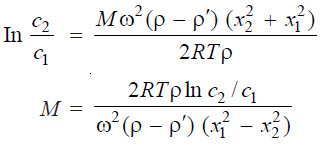Macromolecules : Definition and Molecular Weight
– In this topic, we will discuss the Macromolecules : Definition and Molecular Weight
What are Macromolecules?
– Colloidal solutions are formed by aggregation of atoms or molecules to give particles of colloidal size.
– Macromolecules are substances which are themselves composed of giant molecules and dissolve in a solvent to yield colloidal solutions directly.
– These giant molecules are termed macromolecules.
– The dimensions of the macromolecules fall in a range between 10Å and 10,000Å.
– Proteins (gelatin), synthetic polymers (plastics), synthetic rubber, cellulose and starch all possess macromolecules.
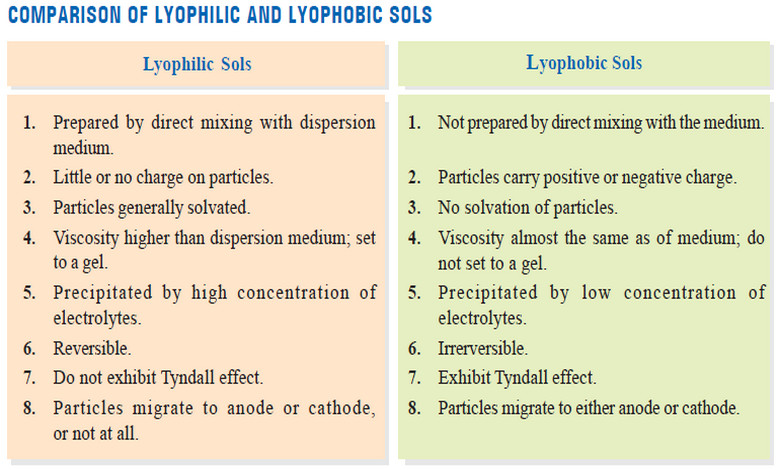
– Solutions of macromolecules behave like reversible colloids or lyophilic sols.
– They show a weak Tyndall effect and possess high viscosity.
– Macromolecules in solution do not carry an electric charge and do not show electrophoresis.
Molecular weight of Macromolecules
– The molecular weight is an important property of polymeric substances such as proteins, polymers (plastics, starch) and other macromolecules.
– Generally, molecules of a protein or a polymer may not be of the same size.
– Therefore all the experimental methods of molecular weight determination will give some kind of an average value.
– Two types of average molecular weights have been defined
(1) Number average Molecular weight.
– It is defined as :
– niMi stands for the weight of macromolecules numbering ni and having molecular weight Mi.
– The experimental methods based on properties which depend on the number of particles present e.g., osmotic pressure, yield number average molecular weight.
(2) Weight average Molecular weight.
It is defined as :
– where m1, m2, etc. represent mass of macromolecules having molecular weights M1, M2, etc. respectively.
– Since
– Molecular weights determined by methods based on properties dependent on the mass of the particles are the weight average molecular weights.
– According to the definitions set out here, .
– The two are equal only when all particles are identical in weight
Solved Problem on Macromolecules
– A polymer mixture contains two polymers, one having molecular weight 100,000 and the other having molecular weight 60,000. The two components are present in equimolar concentration. Calculate the number average and the weight average molecular weights.
Solution:
– This is the number average molecular weight of the polymer mixture.
– Thus the weight average molecular weight is 85,000
Determination of Molecular weight of Macromolecules
– There are a number of methods available for the determination of molecular weight of macromolecules.
– Here, we will discuss the more important ones.
(1) Osmotic Pressure Method
– The van’t Hoff equation for dilute solutions may be written as:
– where p = osmotic pressure, atm ; c = concentration of solution gl–1; R = gas constant, 0.08205 l atm deg–1 mol–1; T = kelvin temperature ; M = molecular weight of the solute (polymer).
– In actual determination of molecular weight of a high polymer, osmotic pressure (p) of a series of
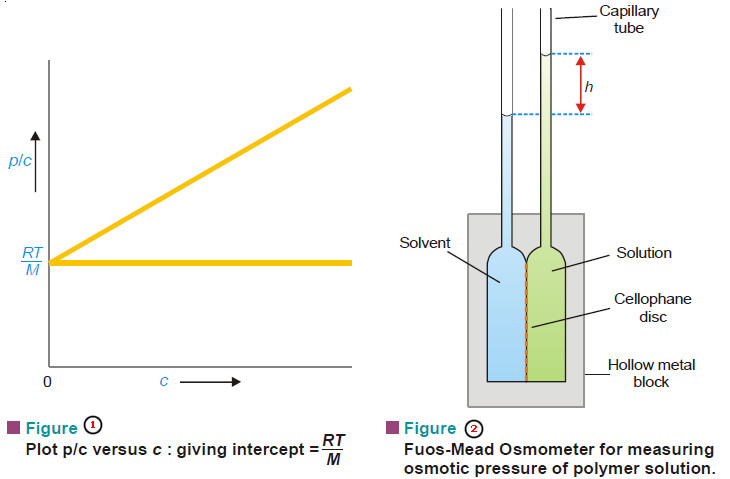
small concentrations (c) is measured with the help of a special Osmometer shown in Fig. 2.
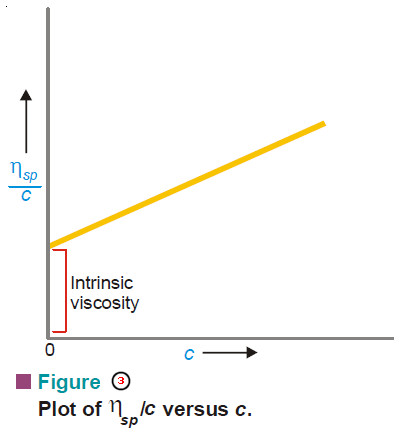
– The plot of p/c against c is a straight line (Fig. 1).
– It is extrapolated to zero concentration.
– This gives RT/M as the intercept from which the molecular weight can be calculated.
Fuos-Mead Osmometer
– It is a modern device for measuring the osmotic pressure of polymer solutions (Fig. 2).
– It consists of two hollow metal blocks holding a cellophane disc in between
– Each block carries a capillary tube.
– The hollow metal compartments are charged with solvent and solution through the side-tubes (not shown).
– Osmosis occurs across the semipermeable membrane (cellophane disc).
– The height of the solution in the capillary (h) is read off differentially to eliminate surface tension effect.
(2) Viscosity method
– It is a very convenient method for determining the molecular weights of macromolecules in solution.
– The addition of macromolecules to a solvent increases its viscosity over that of pure solvent.
– The relative viscosity of a solution of a polymer, denoted by ηr , is given by the expression:
– where η is viscosity of solution and η0 that of the solvent at the same temperature.
– The specific viscosity, denoted by ηsp , is given by:
– In terms of (1) and (2), the intrinsic viscosity is defined as:
– where c is the concentration of the solute.
– The plot ηsp/c against η gives a straight line.
– Extrapolation to c = 0 yields the intrinsic viscosity.
– It was shown by staudinger that an empirical relationship exists between intrinsic viscosity [η] and the molecular weight [M] of the high polymer.
– where k and a are constants for a specific polymer in a specific solvent.
– Once k and a are known for a polymersolvent combination, M may be calculated from a determination of the value of intrinsic viscosity.
– The viscosity measurements yield the weight average molecular weight of a macromolecular substance.
(3) Svedberg’s Sedimentation method
– The rate of selling or sedimentation of polymer particles under the influence of gravitation force is very slow.
– Svedberg devised a centrifugal machine with the help of which macromolecules could be sedimented with speed.
– As a result, the particles move down in the containing tube. This causes a concentration gradient in the tube.
– The rate, dx/dt, at which the macromolecules sediment, is given in terms of the sedimentation constant S by the following expression :
– where x is the distance of the solute species from the centre of rotation and ω is the angular velocity.
– The sedimentation constant, S, is related to the molecular weight of the polymer by the
expression :
– where ρ and ρ’ are the densities of the solvent and solute respectively.
– The rate at which sedimentation occurs, determined experimentally, can thus be used for finding the molecular weight of macromolecules.
– Since the rate of sedimentation depends on the mass of the particle undergoing sedimentation, the molecular weight obtained by this method is the weight average molecular weight,
(4) Sedimentation Equilibrium method
– This method for determining molecular weight of a high polymer is quicker and convenient compared to method (3).
– If a sol is whirled sufficiently long in an ultracentrifuge, a stage is reached at which the sol no longer settles.
– At this stage an equilibrium is reached between the centrifugal force and diffusion of the material in a direction opposite to the centrifugal force.
– If c1 and c2 be the concentrations of the particles at points x1 and x2 cm from the centre of rotation, the molecular weight, M, of the high polymer is given by the relation:
– By determining the concentrations c1 and c2 at the two levels x1 and x2 in the settling cell at sedimentation equilibrium, M can be calculated.