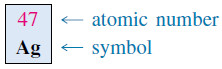Atoms and Molecules
Democritus’s ideas about atoms
– The Greek philosopher Democritus (470–400 BC) suggested that all matter is composed of tiny, discrete, indivisible particles that he called atoms.
– His ideas, based entirely on philosophical speculation rather than experimental evidence, were rejected for 2000 years.
– By the late 1700s, scientists began to realize that the concept of atoms provided an explanation for many experimental observations about the nature of matter.
– By the early 1800s, the Law of Conservation of Matter and the Law of Definite Proportions were both accepted as general descriptions of how matter behaves.
Dalton’s ideas about atoms
– John Dalton (1766–1844), an English schoolteacher, tried to explain why matter behaves in such systematic ways as those expressed here.
– In 1808, he published the first “modern” ideas about the existence and nature of atoms.
– Dalton’s explanation summarized and expanded the nebulous concepts of early philosophers and scientists; more importantly, his ideas were based on reproducible experimental results of measurements by many scientists.
– These ideas form the core of Dalton’s Atomic Theory, one of the highlights in the history of scientific thought.
– In condensed form, Dalton’s ideas may be stated as follows:
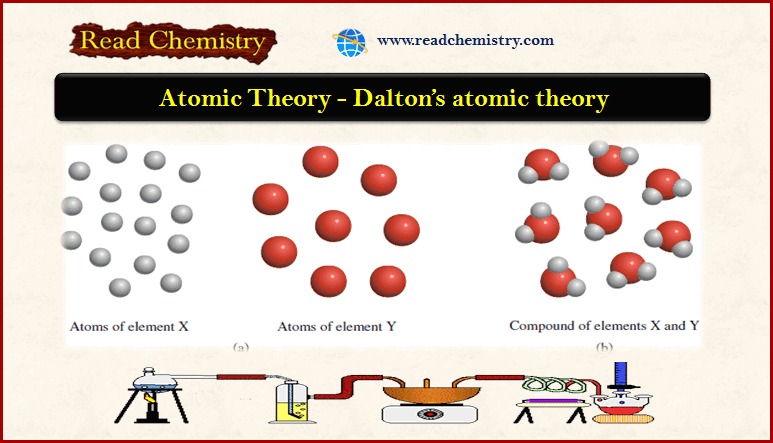
(1) An element is composed of extremely small, indivisible particles called atoms.
(2) All atoms of a given element have identical properties that differ from those of other elements.
(3) Atoms cannot be created, destroyed, or transformed into atoms of another element.
(4) Compounds are formed when atoms of different elements combine with one another in small whole-number ratios.
(5) The relative numbers and kinds of atoms are constant in a given compound.
– Dalton believed that atoms were solid, indivisible spheres, an idea we now reject. But he showed remarkable insight into the nature of matter and its interactions.
– Some of his ideas could not be verified (or refuted) experimentally at the time. They were based on the limited experimental observations of his day.
– Even with their shortcomings, Dalton’s ideas provided a framework that could be modified and expanded by later scientists.
– Thus John Dalton is often considered to be the father of modern atomic theory.
– The smallest particle of an element that maintains its chemical identity through all chemical and physical changes is called an atom (Figure 1).
Basic Components of atom
– we shall study the structure of the atom in detail; let us simply summarize here the main features of atomic composition.
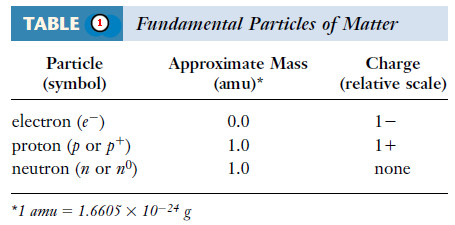
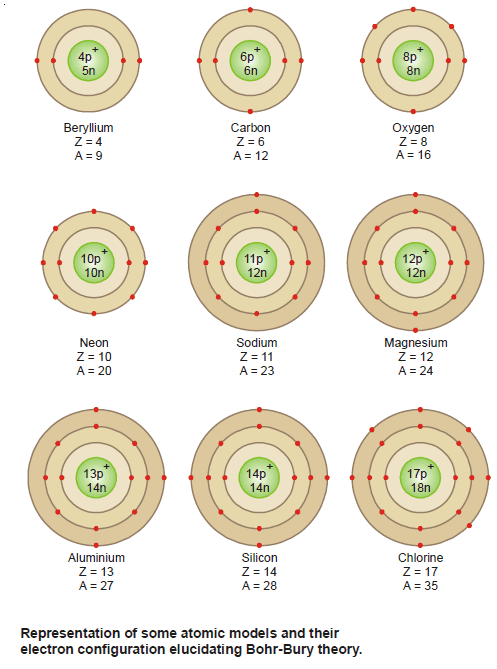
– Atoms, and therefore all matter, consist principally of three fundamental particles: electrons, protons, and neutrons.
– These are the basic building blocks of atoms.
– The masses and charges of the three fundamental particles are shown in Table (1).
– The masses of protons and neutrons are nearly equal, but the mass of an electron is much smaller.
– Neutrons carry no charge.
– The charge on a proton is equal in magnitude, but opposite in sign, to the charge on an electron.
– Because atoms are electrically neutral, an atom contains equal numbers of electrons and protons.
Atomic number
– The atomic number (symbol is Z) of an element is defined as the number of protons in the nucleus.
– In the periodic table, elements are arranged in order of increasing atomic numbers.
– These are the red numbers above the symbols for the elements in the periodic table on the inside front cover.
– For example, the atomic number of silver is 47.
What are Molecules?
– A molecule is the smallest particle of an element or compound that can have a stable independent existence.
– In nearly all molecules, two or more atoms are bonded together in very small, discrete units (particles) that are electrically neutral.
– Individual oxygen atoms are not stable at room temperature and atmospheric pressure.
– Single atoms of oxygen mixed under these conditions quickly combine to form pairs.
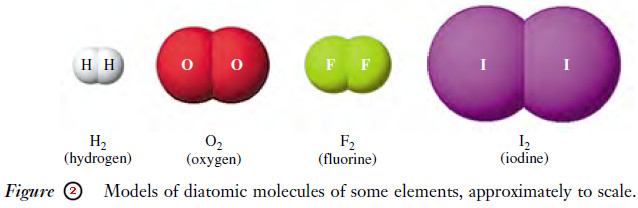
– The oxygen with which we are all familiar is made up of two atoms of oxygen; it is a diatomic molecule, O2.
– Hydrogen, nitrogen, fluorine, chlorine, bromine, and iodine are other examples of diatomic molecules (Figure 2).
– Some other elements exist as more complex molecules.
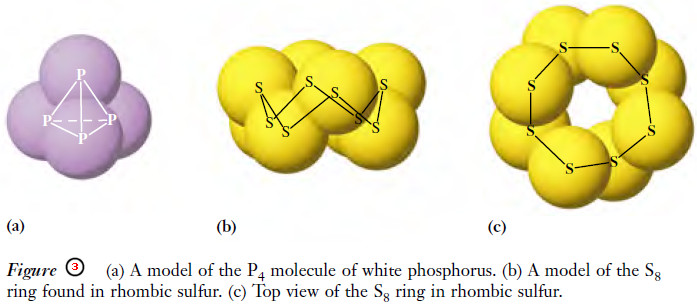
– One form of phosphorus molecules consists of four atoms, and sulfur exists as eight-atom molecules at ordinary temperatures and pressures.
– Molecules that contain two or more atoms are called polyatomic molecules (Figure 3).
– In modern terminology, O2 is named dioxygen, H2 is dihydrogen, P4 is tetraphosphorus, and so on.
– Even though such terminology is officially preferred, it has not yet gained wide acceptance.
– Most chemists still refer to O2 as oxygen, H2 as hydrogen, P4 as phosphorus, and so on.
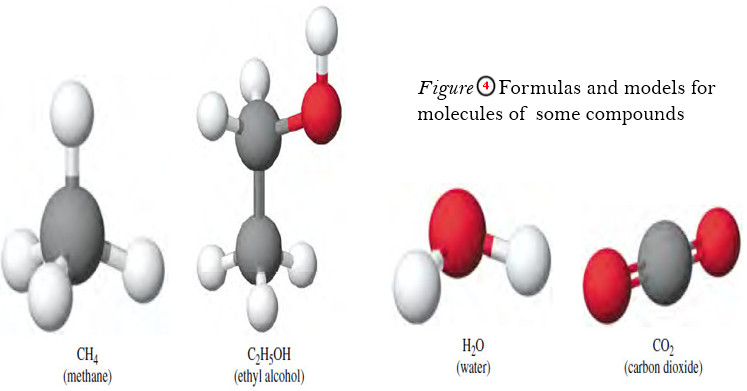
– Molecules of compounds are composed of more than one kind of atom.
– A water molecule consists of two atoms of hydrogen and one atom of oxygen.
– A molecule of methane consists of one carbon atom and four hydrogen atoms.
– The shapes of a few molecules are shown in Figure (4).
– Atoms are the components of molecules, and molecules are the stable forms of many elements and compounds.
– We are able to study samples of compounds and elements that consist of large numbers of atoms and molecules.
– With the scanning tunnelling microscope it is now possible to “see” atoms.
– It would take millions of atoms to make a row as long as the diameter of the period at the end of this sentence.
References
- Principles of Inorganic Chemistry / Brian W. Pfennig / 1st ed, 2015 /John Wiley & Sons, Inc/ USA.
- Inorganic Chemistry /Peter Atkins, Tina Overton, Jonathan Rourkel, Mark Weller, Fraser Armstrong, Mike Hagerman / 6th ed, 2014 /W. H. Freeman and Company/ New York, USA.
-