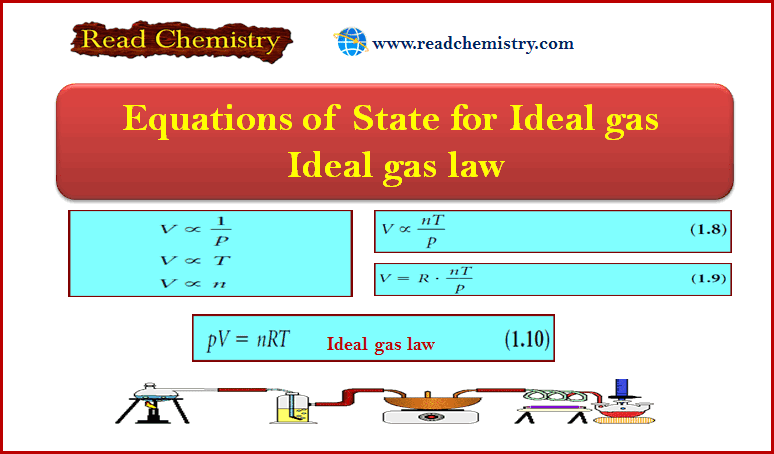
Chemical Formula – Structural Formula
Chemical Formula
– The chemical formula for a substance shows its chemical composition.
– This represents the elements present as well as the ratio in which the atoms of the elements occur.
– The formula for a single atom is the same as the symbol for the element. Thus, Na can represent a single sodium atom.
– It is unusual to find such isolated atoms in nature, with the exception of the noble gases. Ex: (He, Ne, Ar, Kr, Xe, and Rn).
– A subscript following the symbol of an element indicates the number of atoms in a molecule.
– For instance, F2 indicates a molecule containing two fluorine atoms, and P4 a molecule containing four phosphorus atoms.
– Some elements exist in more than one form. Familiar examples include (1) oxygen, found as O2 molecules, and ozone, found as O3 molecules, and (2) two crystalline forms of carbon—diamond and graphite
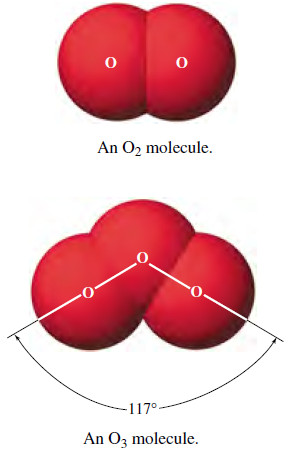
– Different forms of the same element in the same physical state are called allotropic modifications, or allotropes.
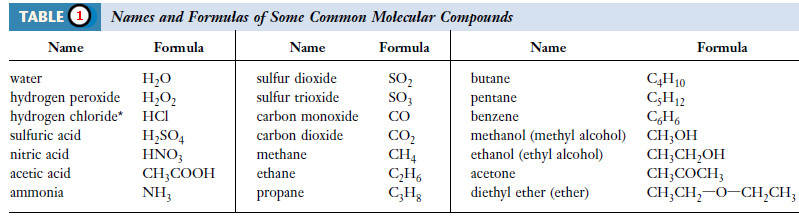
– Compounds contain two or more elements in chemical combination in fixed proportions.
– Many compounds exist as molecules (Table 1).
– Hence, each molecule of hydrogen chloride, HCl, contains one atom of hydrogen and one atom of chlorine; each molecule of carbon tetrachloride, CCl4, contains one carbon atom and four chlorine atoms.
– An aspirin molecule, C9H8O4, contains nine carbon atoms, eight hydrogen atoms, and four oxygen atoms.
– Many of the molecules found in nature are organic compounds.
– Organic compounds contain CXC or CXH bonds or both.
– Eleven of the compounds listed in Table (1) are organic compounds (acetic acid and the last ten entries).
– All of the other compounds in the table are inorganic compounds.
– Some groups of atoms behave chemically as single entities.
– For instance, an oxygen atom that is bonded to a hydrogen atom and also to a carbon atom that is bonded to three other atoms forms the reactive combination of atoms known as the alcohol group or molecule.
– In formulas of compounds containing two or more of the same group, the group formula is enclosed in parentheses.
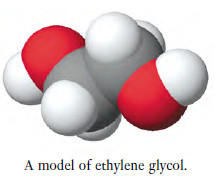
– Thus, ethylene glycol contains two alcohol groups and its formula is C2H4(OH)2 (see structure in the margin).When you count the number of atoms in this molecule from its formula, you must multiply the numbers of hydrogen and oxygen atoms in the OH group by 2. There are two carbon atoms, six hydrogen atoms and two oxygen atoms in a molecule of ethylene glycol.
– Compounds were first recognized as distinct substances because of their different physical properties and because they could be separated from one another by physical methods.
– Once the concept of atoms and molecules was established, the reason for these differences in properties could be understood: Two compounds differ from each other because their molecules are different.
– Conversely, if two molecules contain the same number of the same kinds of atoms, arranged the same way, then both are molecules of the same compound. Thus, the atomic theory explains the Law of Definite Proportions.
– This law, also known as the Law of Constant Composition, can now be extended to include its interpretation in terms of atoms.
Different pure samples of a compound always contain the same elements in the same proportion by mass; this corresponds to atoms of these elements combined in fixed numerical ratios.
Disadvantage of Chemical formula
– So we see that for a substance composed of molecules, the chemical formula gives the number of atoms of each type in the molecule.
– But this formula does not express the order in which the atoms in the molecules are bonded together.
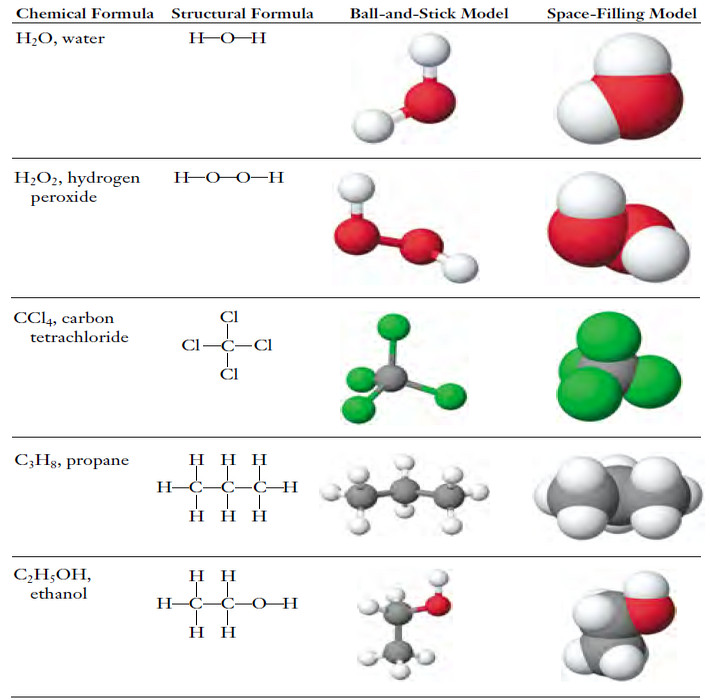
Structural Formula
– The structural formula shows the order in which atoms are connected.
– The lines connecting atomic symbols represent chemical bonds between atoms.
– The bonds are actually forces that tend to hold atoms at certain distances and angles from one another.
– For instance, the structural formula of propane shows that the three C atoms are linked in a chain, with three H atoms bonded to each of the end C atoms and two H atoms bonded to the center C.
Ball-and-stick and space-filling models
– Ball-andstick molecular models and space-filling molecular models help us to see the shapes and relative sizes of molecules.
– These four representations are shown in the following Figure.
– The balland- stick and space-filling models show:
(1) the bonding sequence, that is the order in which the atoms are connected to each other, and
(2) the geometrical arrangements of the atoms in the molecule.
– As we shall see later, both are extremely important because they determine the properties of compounds.
References
- Principles of Inorganic Chemistry / Brian W. Pfennig / 1st ed, 2015 /John Wiley & Sons, Inc/ USA.
- Inorganic Chemistry /Peter Atkins, Tina Overton, Jonathan Rourkel, Mark Weller, Fraser Armstrong, Mike Hagerman / 6th ed, 2014 /W. H. Freeman and Company/ New York, USA.
-