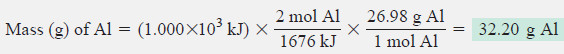Stoichiometry of Thermochemical Equation
Stoichiometry of Thermochemical Equation
– A thermochemical equation is a balanced equation that includes the heat of reaction (ΔHrxn).
– Keep in mind that the ΔHrxn value shown refers to the amounts (moles) of substances and their states of matter in that specific equation.
– The enthalpy change of any process has two aspects:
(1) Sign
– The sign of ΔH depends on whether the reaction is exothermic (-) or endothermic (+).
– A forward reaction has the opposite sign of the reverse reaction.
– Decomposition of 2 mol of water to its elements (endothermic):
2H2O(l) → 2H2(g) + O2(g) ΔHrxn = 572 kJ
– Formation of 2 mol of water from its elements (exothermic):
2H2(g) + O2(g) → 2H2O(l) ΔHrxn= -572 kJ
(2) Magnitude
– The magnitude of ΔH is proportional to the amount of substance reacting.
– Formation of 1 mol of water from its elements (half the amount in the preceding equation):
H2(g) + 1/2 O2(g) → H2O(l) ΔHrxn= -286 kJ
– Note that, in thermochemical equations, we often use fractional coefficients to specify the magnitude of ΔHrxn for a particular amount of substance.
– Moreover, in a particular reaction, a certain amount of substance is thermochemically equivalent to a certain quantity of energy.
– In the reaction just shown,
286 kJ is thermochemically equivalent to 1 mol of H2(g)
and 286 kJ is thermochemically equivalent to mol of O2(g)
286 kJ is thermochemically equivalent to 1 mol of H2O(l)
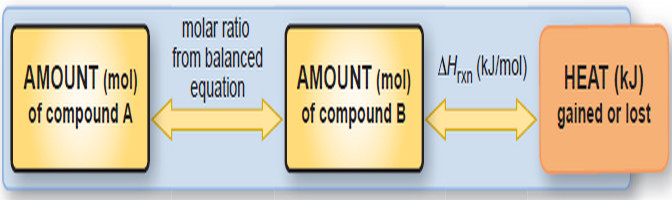
– Just as we use stoichiometrically equivalent molar ratios to find amounts of substances, we use thermochemically equivalent quantities to find the heat of reaction for a given amount of substance.
– Also, just as we use molar mass (in g/mol of substance) to convert moles of a substance to grams, we use the heat of reaction (in kJ/mol of substance) to convert moles of a substance to an equivalent quantity of heat (in kJ).
– The followingFigure shows this new relationship, and the following solved Problem applies it.
– Using the Heat of Reaction (ΔHrxn) to Find Amounts
The major source of aluminum in the world is bauxite (mostly aluminum oxide). Its thermal decomposition can be represented by:
Al2O3(s) → 2Al(s) + 3/2 O2(g) ΔHrxn =1676 kJ
If aluminum is produced this way , how many grams of aluminum can form when 1.000×103 kJ of heat is transferred?
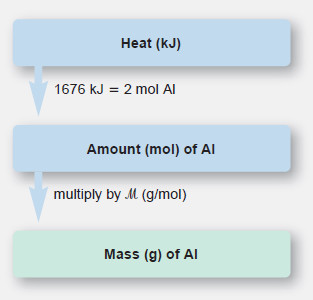
Plan:
– From the balanced equation and the enthalpy change, we see that 2 mol of Al forms when 1676 kJ of heat is absorbed.
– With this equivalent quantity, we convert the given kJ transferred to moles formed and then convert moles to grams.
Solution:
Combining steps to convert from heat transferred to mass of Al:
Check:
The mass of aluminum seems correct: ≈1700 kJ forms about 2 mol of Al (54 g), so 1000 kJ should form a bit more than half that amount (27 g).
Comment:
– In practice, aluminum is not obtained by heating but by supplying electrical energy.
– Because ΔH is a state function, however, the total energy required for this chemical change is the same no matter how it occurs.
Summary
– A thermochemical equation shows the balanced equation and its ΔHrxn.
– The sign of ΔH for a forward reaction is opposite that for the reverse reaction.
– The magnitude of ΔH depends on the amount and physical state of the substance reacting and the ΔH per mole of substance.
– We use the thermochemically equivalent amounts of substance and heat from the balanced equation as conversion factors to find the quantity of heat when a given amount of substance reacts.
References
- Principles of Inorganic Chemistry / Brian W. Pfennig / 1st ed, 2015 /John Wiley & Sons, Inc/ USA.
- Principles of general Chemistry / Martin S. Silberberg / 2nd ed, 2010 /The McGraw-Hill Companies, Inc./ USA.
- Inorganic Chemistry /Peter Atkins, Tina Overton, Jonathan Rourkel, Mark Weller, Fraser Armstrong, Mike Hagerman / 6th ed, 2014 /W. H. Freeman and Company/ New York, USA.
-