-
Organic Chemistry
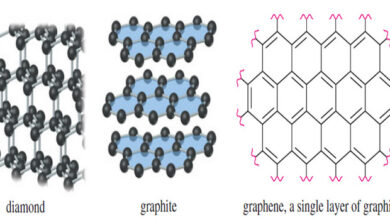
Aromatic Allotropes of Carbon
– In this subject, we will talk about Aromatic Allotropes of Carbon. Aromatic Allotropes of Carbon What do you get…
Read More » -
Organic Chemistry
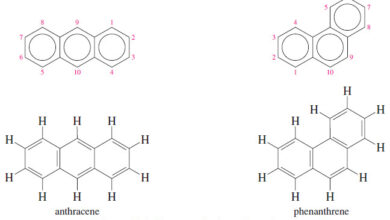
Polynuclear Aromatic Hydrocarbons
Polynuclear Aromatic Hydrocarbons – The polynuclear aromatic hydrocarbons (abbreviated PAHs or PNAs) are composed of two or more fused benzene…
Read More » -
Organic Chemistry
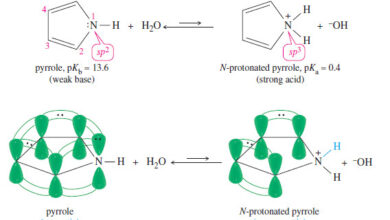
Heterocyclic Aromatic Compounds
Heterocyclic Aromatic Compounds – Nitrogen, oxygen, and sulfur are the most common heteroatoms in heterocyclic aromatic compounds. – The criteria…
Read More » -
Organic Chemistry
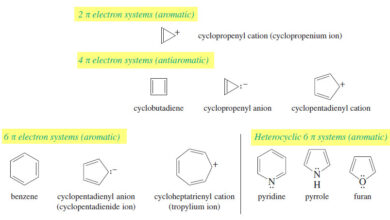
Aromatic, Antiaromatic, and Nonaromatic Compounds
Aromatic, Antiaromatic, and Nonaromatic Compounds – Our working definition of aromatic compounds has included cyclic compounds containing conjugated double bonds…
Read More » -
Organic Chemistry
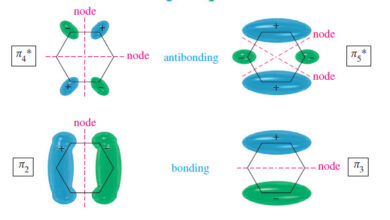
The Molecular Orbitals of Benzene
In this subject, we will talk about The Molecular Orbitals of Benzene The Molecular Orbitals of Benzene – Visualizing benzene…
Read More » -
Organic Chemistry
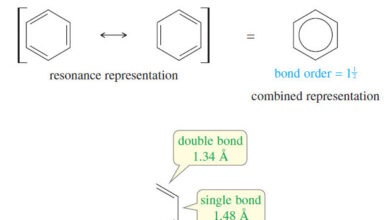
The Structure and Properties of Benzene
In this subject, we we will talk about The Discovery of Benzene and The Structure and Properties of Benzene. The…
Read More » -
General Chemistry
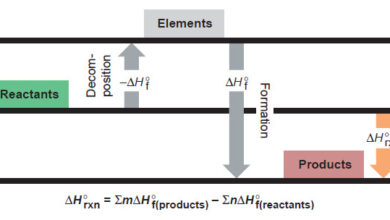
Standard Heats of Reaction (ΔH°rxn)
In this subject, we talk about Standard Heats of Reaction (ΔH°rxn) Standard Heats of Reaction (ΔH°rxn) – In this subject,…
Read More » -
General Chemistry
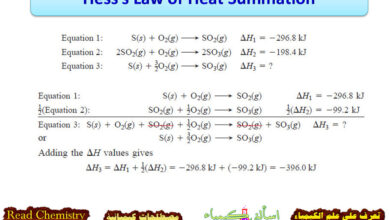
Hess’s Law of Heat Summation
In this subject, we will discuss Hess’s Law of Heat Summation Hess’s Law of Heat Summation – Many reactions are…
Read More » -
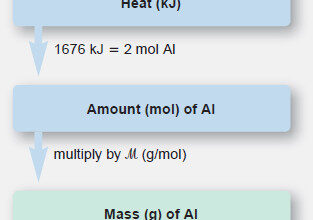
General Chemistry
Stoichiometry of Thermochemical Equation
Stoichiometry of Thermochemical Equation – A thermochemical equation is a balanced equation that includes the heat of reaction (ΔHrxn). –…
Read More » -
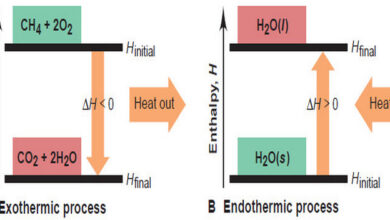
General Chemistry
Enthalpy: Heats of Reaction and Chemical Change
In this subject, we will discuss the Enthalpy: Heats of Reaction and Chemical Change. Enthalpy: Heats of Reaction and Chemical…
Read More » -
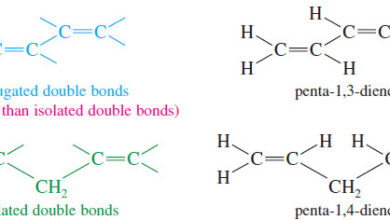
Organic Chemistry
Stabilities of Dienes
– In this topic, we will talk about Stabilities of Dienes What are Dienes? – Double bonds can interact with…
Read More » -
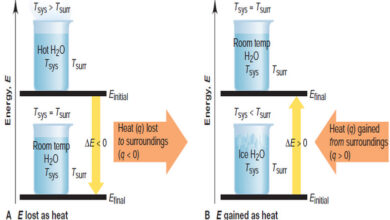
General Chemistry
Forms of Energy and Their Interconversion
Forms of Energy and Their Interconversion – we discussed before the facts that all energy is either potential or kinetic…
Read More » -
Organic Chemistry
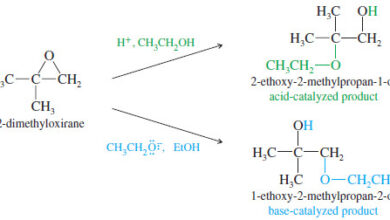
Ring Opening of Epoxides
– In this topic, we will talk aboutAcid-Catalyzed Ring Opening of Epoxides, Base-Catalyzed Ring Opening of Epoxides, and Orientation of…
Read More » -
General Chemistry
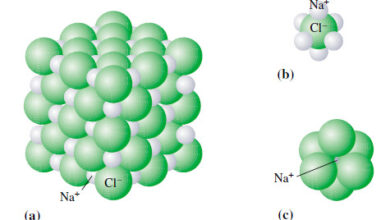
Ions and ionic compounds
– In this topic, we will discuss definition of The Ions and ionic compounds Ions and ionic compounds – So…
Read More » -
Physical Chemistry
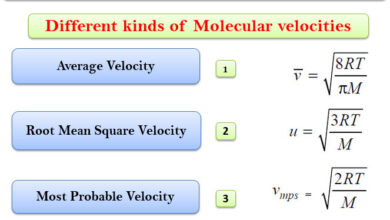
Distribution of Molecular velocities
– In this topic, we will discuss Distribution of Molecular velocities. Distribution of Molecular velocities – While deriving Kinetic Gas…
Read More » -
Biochemistry
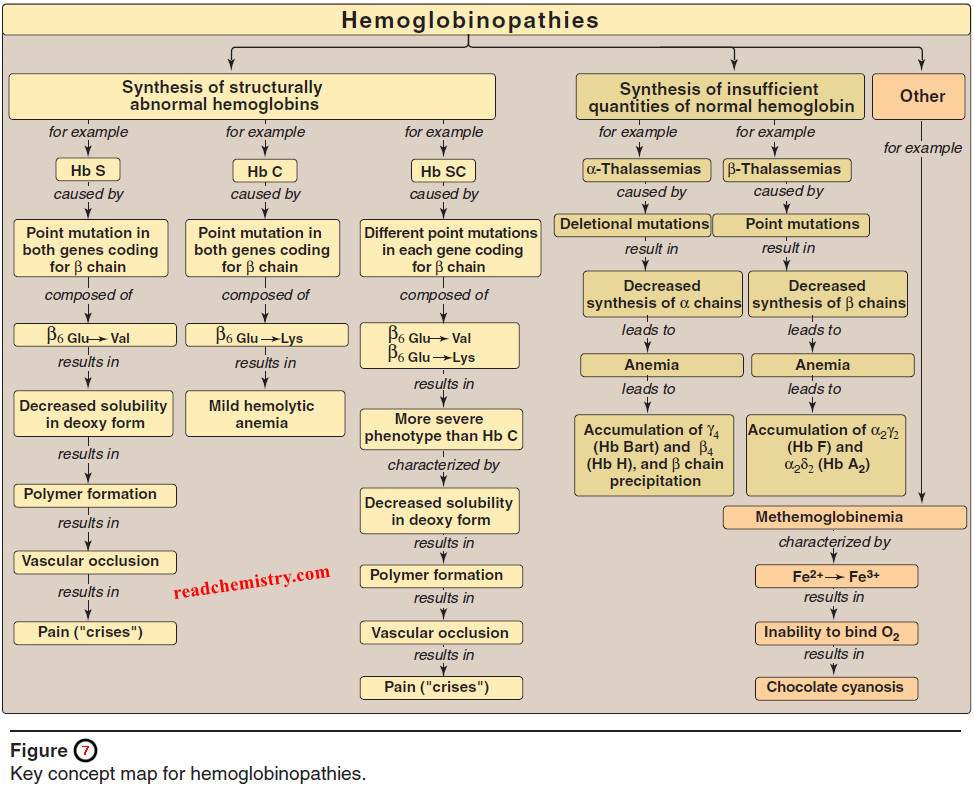
Hemoglobinopathies
Hemoglobinopathies – Hemoglobinopathies have traditionally been defined as a family of genetic disorders caused by production of a structurally abnormal…
Read More » -
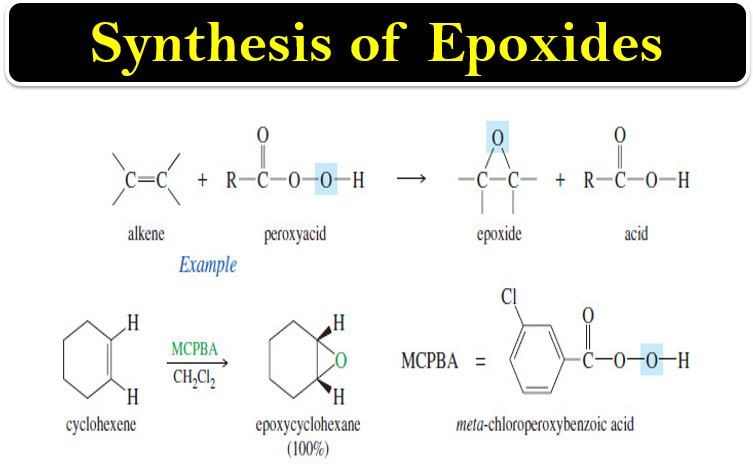
Organic Chemistry
Synthesis of Epoxides
– In this topic, we will talk about Synthesis of Epoxides by two methods: Peroxyacid epoxidation and Base-promoted cyclization of…
Read More » -
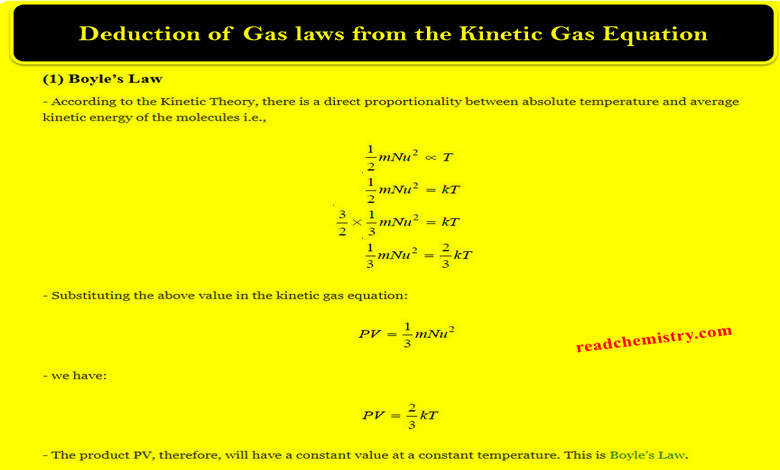
Physical Chemistry
Deduction of Gas laws from the Kinetic Gas Equation
– In this topic, we will talk about Deduction of Gas laws from the Kinetic Gas Equation. Assumptions of the…
Read More » -
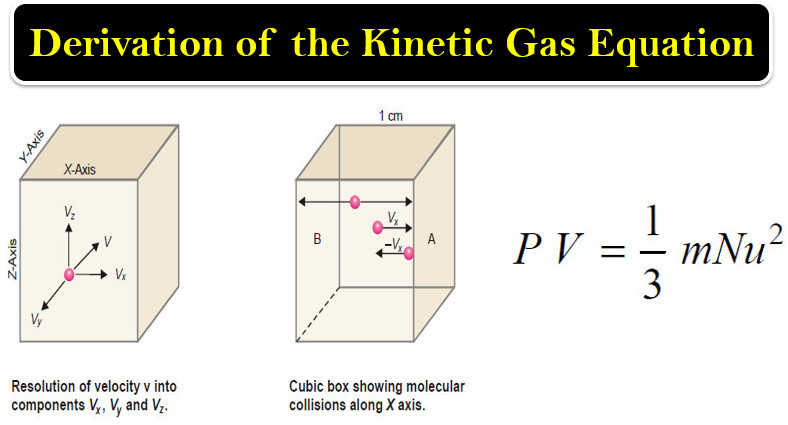
Physical Chemistry
Derivation of the Kinetic Gas Equation
– In this topic, we will talk about Derivation of the Kinetic Gas Equation. Derivation of the Kinetic Gas Equation…
Read More » -
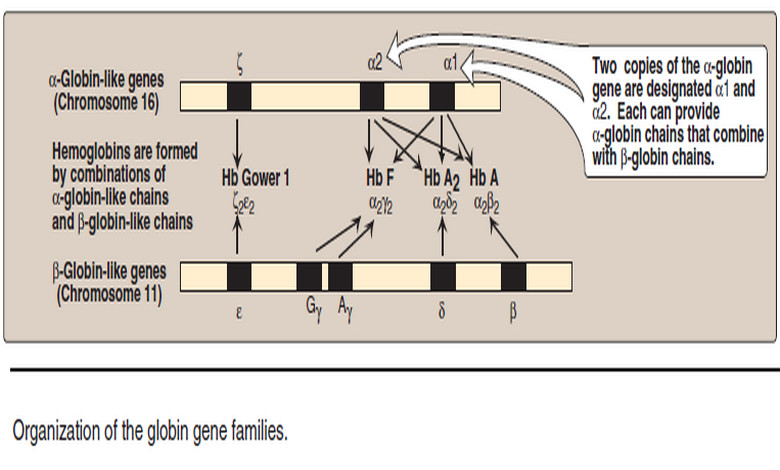
Biochemistry
Organization of the globin genes
– In this topic, we will talk about Organization of the globin genes. Organization of the globin genes – To…
Read More »