-
Physical Chemistry
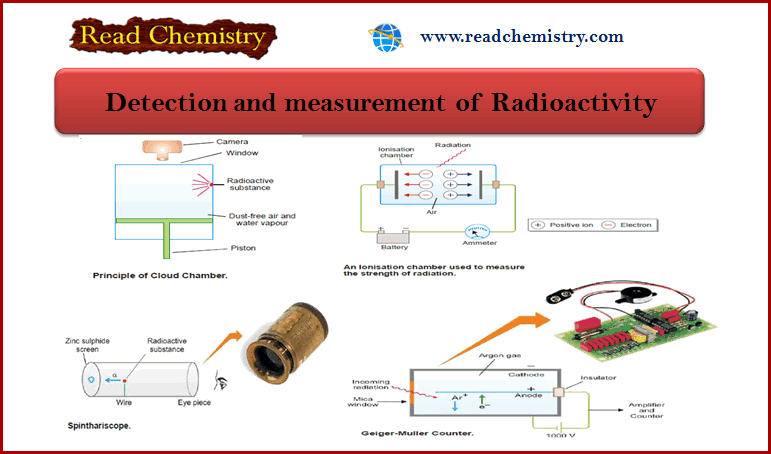
Radioactivity: Detection and Measurement
– In this subject, we will discuss the Detection and measurement of Radioactivity. Detection and measurement of Radioactivity – Radioactivity…
Read More » -
General Chemistry
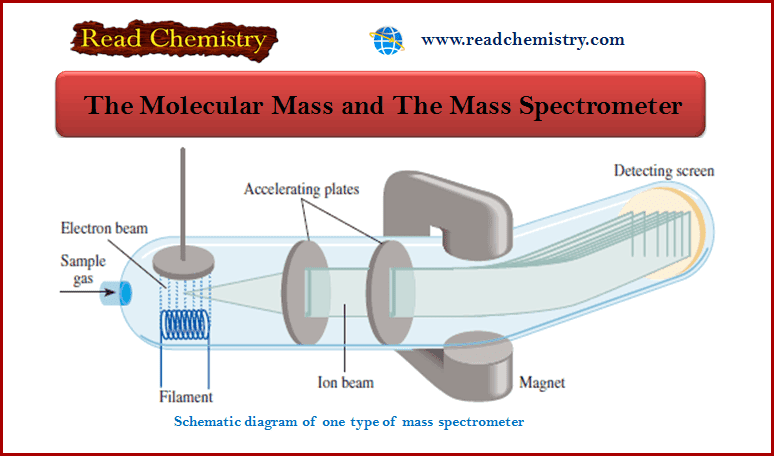
The Molecular Mass and The Mass Spectrometer
Molecular Mass ** If we know the atomic masses of the component atoms, we can calculate the mass of…
Read More » -
General Chemistry
Avogadro’s Number and the Molar Mass of an Element
Avogadro’s Number (NA) ** Atomic mass units provide a relative scale for the masses of the elements. ** But because…
Read More » -
General Chemistry
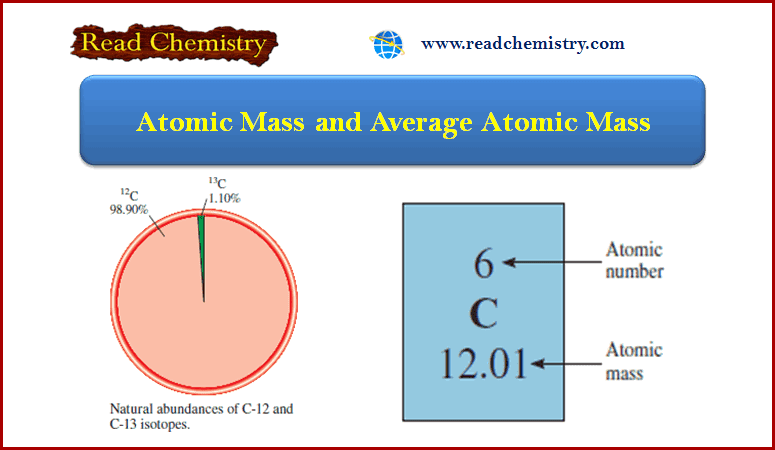
Atomic Mass and Average Atomic Mass: Definition, Calculation
– In this subject, we will discuss the Atomic Mass and Average Atomic Mass: Definition, Formula, and Calculation Atomic Mass…
Read More » -
Physical Chemistry
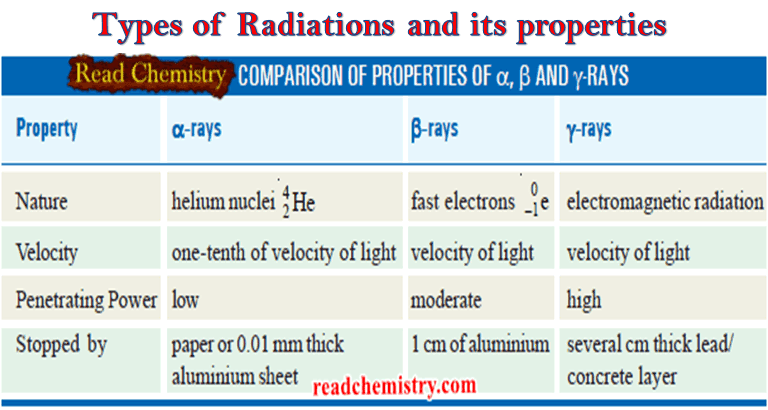
Types of Radiations and its properties
Nuclear reaction ** A nuclear reaction is different from a chemical reaction. ** In a chemical reaction, atoms…
Read More » -
Organic Chemistry
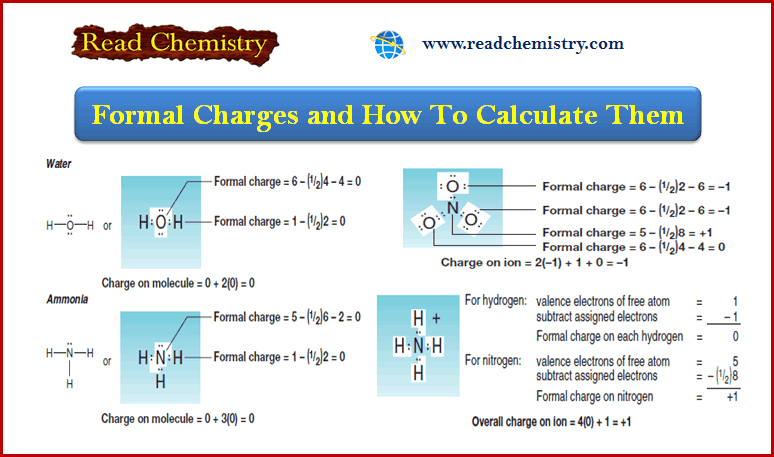
Formal Charge: Definition, Formula, Calculation, Examples
– In this subject, we will discuss the Formal Charge: Definition, Formula, Calculation, Examples Formal Charge and How To Calculate…
Read More » -
General Chemistry
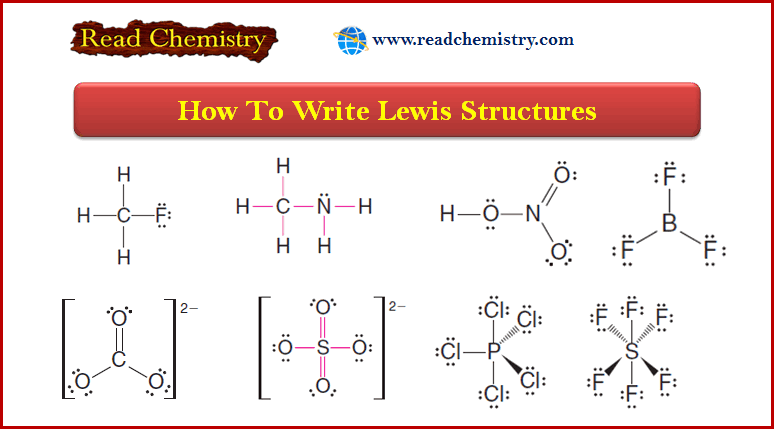
Lewis Structures: Definition, Structural Formula, Examples
– In this subject, we will discuss the Lewis Structures: Definition, Overview, Structural Formula, Examples Definition of Lewis structures –…
Read More » -
General Chemistry
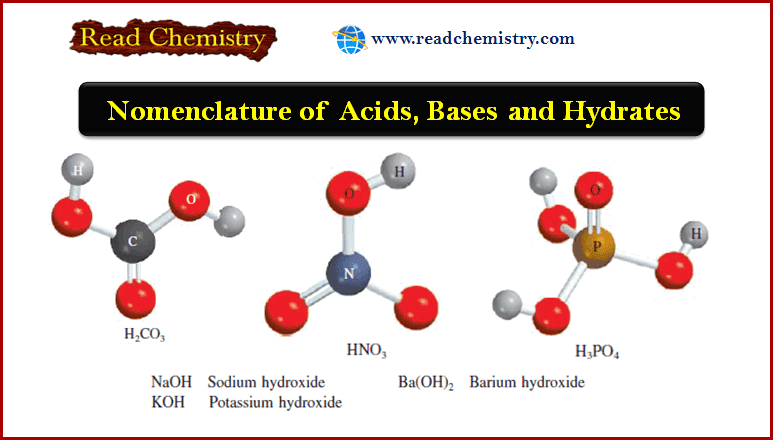
Nomenclature of Acids, Bases and Hydrates
– In this subject, we will discuss the Nomenclature of Acids, Bases and Hydrates Nomenclature of Acids – An acid…
Read More » -
General Chemistry
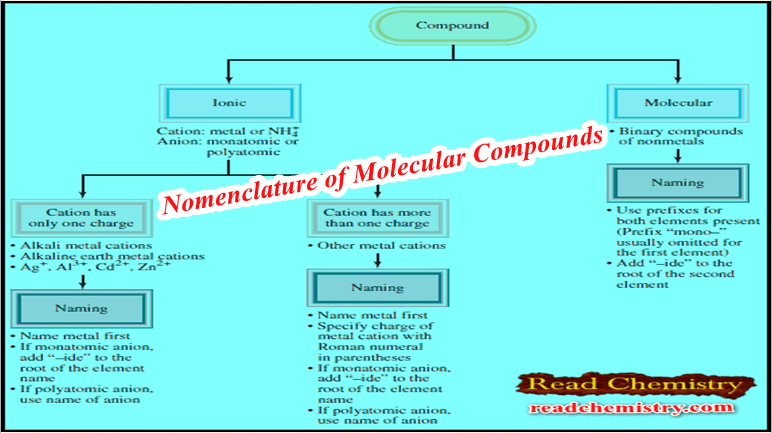
Nomenclature of Molecular Compounds
How to Name Molecular Compounds – Unlike ionic compounds, Molecular compounds contain discrete molecular units. – They are usually composed…
Read More » -
General Chemistry
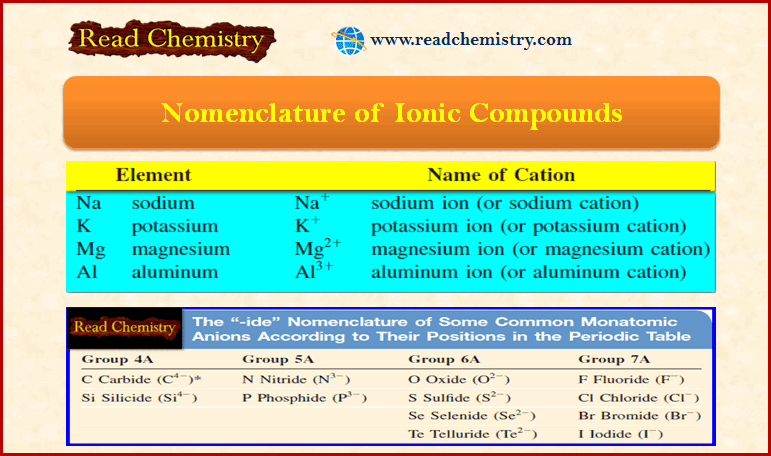
Nomenclature of Ionic Compounds
– In this subject, we will discuss the Nomenclature of Ionic Compounds. Naming Compounds – In addition to using formulas…
Read More » -
General Chemistry
Molecular Formulas and Empirical Formulas: Definitions, Examples
– In this subject, we will discuss Molecular Formulas and Empirical Formulas: Definitions, Examples – Chemists use Chemical formulas to express…
Read More » -
General Chemistry
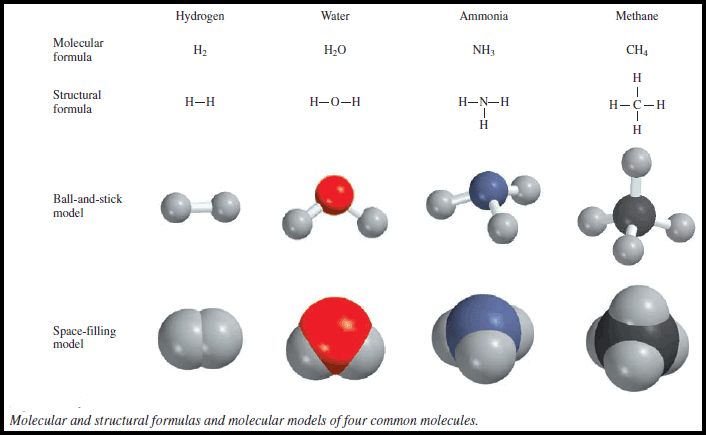
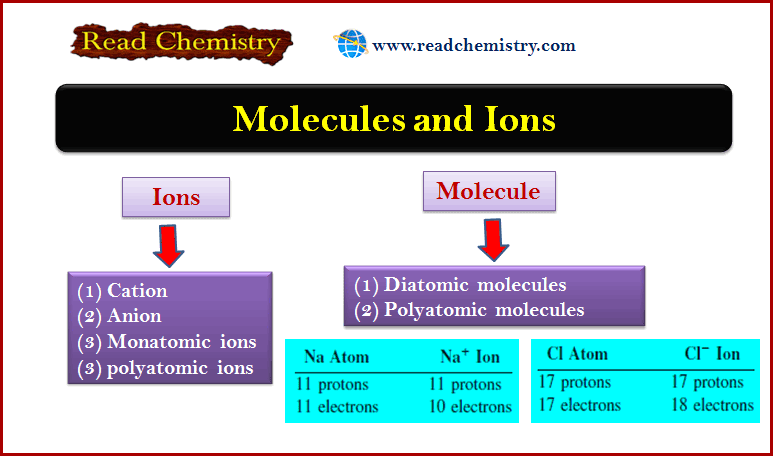
Atoms, Molecules, and Ions: Definition, Types, Examples
– In this subject, we will discuss the Atoms, Molecules, and Ions: Definition, Types, Examples Atoms – Of all the…
Read More » -
General Chemistry
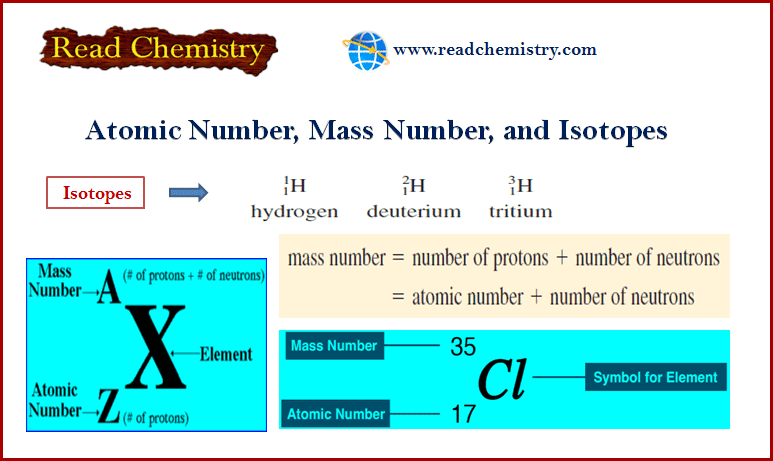
Atomic Number, Mass Number, and Isotopes
– In this subject, we will discuss the Atomic Number, Mass Number, and Isotopes: Definition, Formula, and Calculation Atomic number…
Read More » -
Physical Chemistry
MCQ on: Isotopes, Isobars, and Isotones
MCQ on: Isotopes, Isobars, and Isotones – In this subject, you will find 25 questions and answers MCQ on Isotopes,…
Read More » -
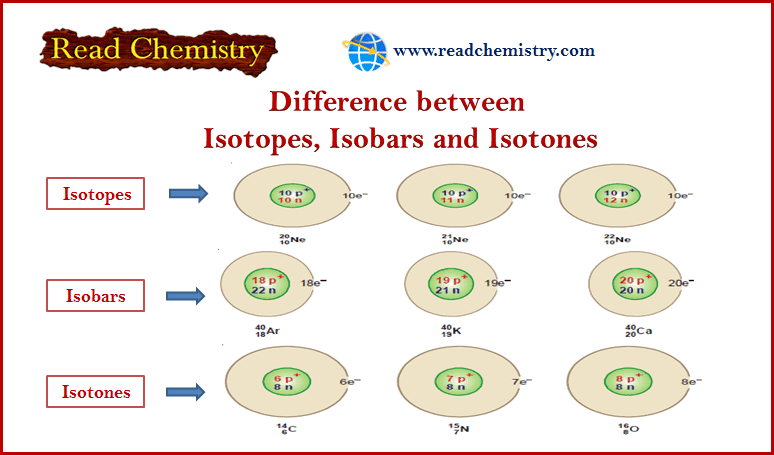
Physical Chemistry
Difference between Isotopes, Isobar, and Isotones
In this subject, the Difference between Isotopes, Isobar, and Isotones will be discussed with some Common Examples for all types.…
Read More » -
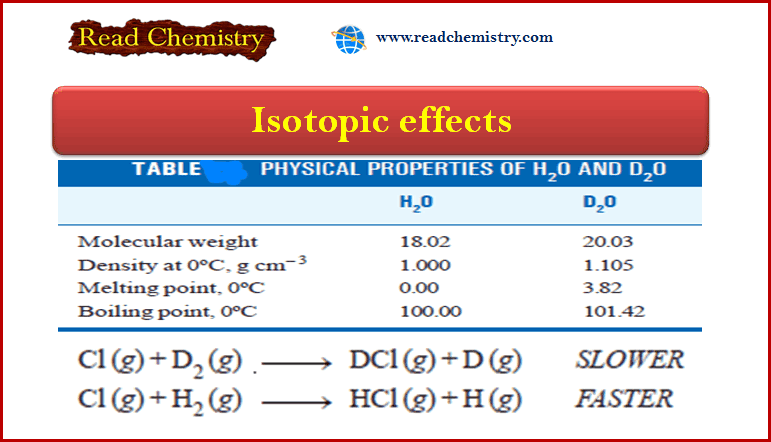
Physical Chemistry
Isotopic effects: Definition, Applications
– In this subject, we will discuss the Isotopic effects: definition, Applications Definition of isotopes Isotopes may be defined as…
Read More » -
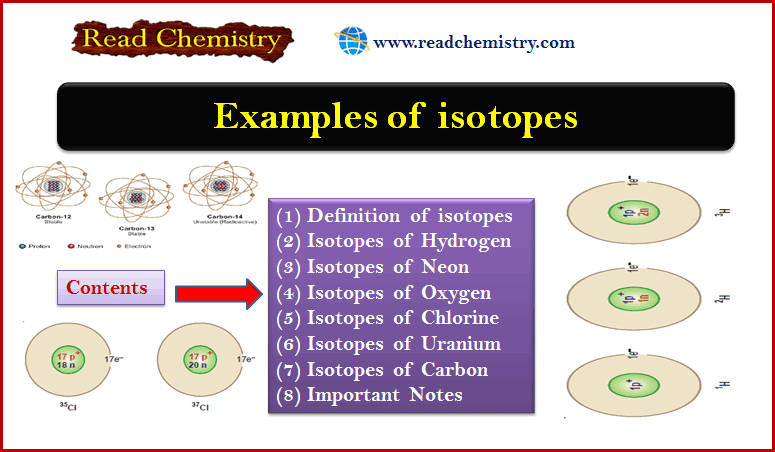
Physical Chemistry
Examples of isotopes
– In this subject, we will discuss 7 Examples of isotopes Definition of isotopes Isotopes may be defined as :…
Read More » -
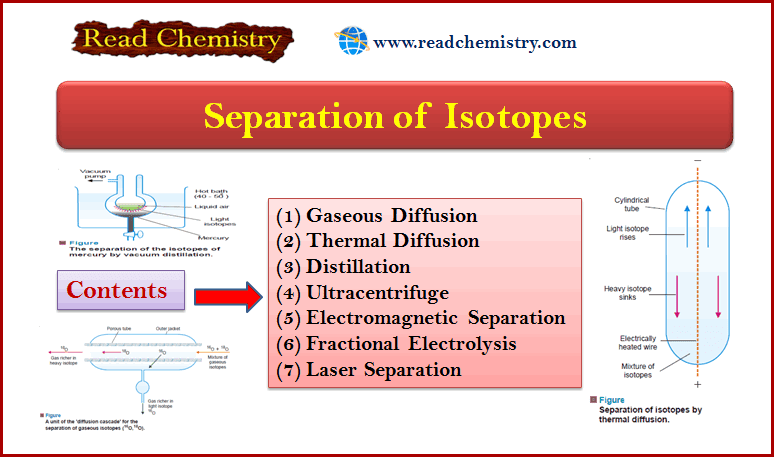
Physical Chemistry
Separation of Isotopes
– In this subject, we will discuss the Separation of Isotopes by seven methods Separation of Isotopes – Since isotopes…
Read More » -
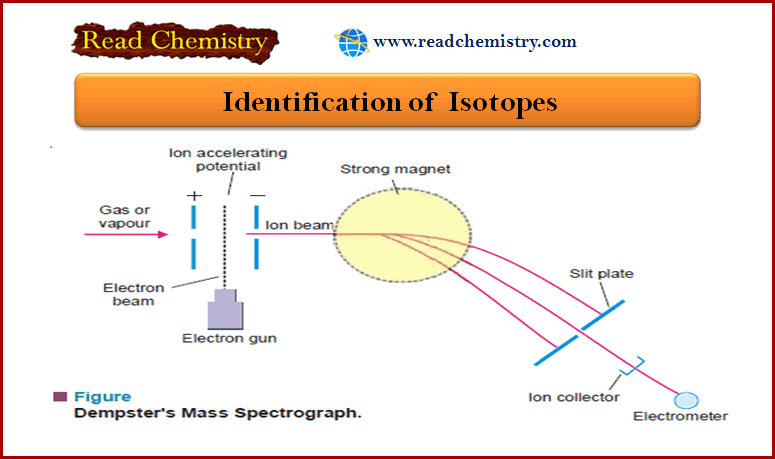
Physical Chemistry
Identification of Isotopes
– In this subject, we will discuss the Identification of Isotopes by Aston’s Mass Spectrograph and Dempster’s Mass Spectrograph Identification…
Read More » -
Physical Chemistry
Isotopes: Definition, representation, Examples
– In this subject, we will discuss the Isotopes: Definition, representation, Examples What are Isotopes? – Contrary to Dalton’s Atomic…
Read More »