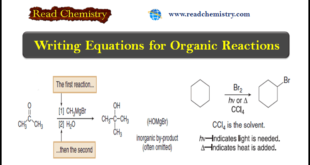
– In this subject, we will discuss Writing Equations for Organic Reactions. Writing Equations for Organic Reactions (1) Like other reactions, equations for organic reactions are usually drawn with a single reaction arrow (→) between the starting material and product, but other conventions make these equations look different from those …
Read More »Filtration and Ignition of Solids
– In this subject, we will discuss the Filtration and Ignition of Solids. – Several techniques and experimental arrangements allow solids to be filtered and ignited with minimal contamination and error. (1) Apparatus for Filtration of Solids (a) Simple Crucibles – Simple crucibles serve only as containers. – Porcelain, aluminum …
Read More »Concise Physical Chemistry book by Donald W.Rogers
– In this subject, we will discuss the free download of Concise Physical Chemistry book by Donald W.Rogers The Preface of Concise Physical Chemistry book – Physical chemistry stands at the intersection of the power and generality of classical and quantum physics with the minute molecular complexity of chemistry …
Read More »Free Download Electrochemistry book (Principles and Applications)
– In this subject, we will discuss Free Download Electrochemistry book (Principles, Methods, and Applications) The Preface of Electrochemistry book – Electrochemistry has undergone significant transformations in the last few decades. – It is not now the province of academics interested only in measuring thermodynamic properties of solutions or of …
Read More »Fundamentals of Chemistry book by Romain Elsair – Free download
– In this subject, we will discuss the free download of Fundamentals of Chemistry book by Romain Elsair. Aim of Fundamentals of Chemistry book – This book addresses first-year students and aims at: (1) Developing further knowledge and understanding of some core scientific concepts and principles (2) Improving ability to …
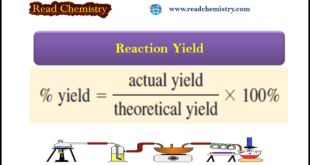
Read More »Reaction Yield – How to Calculate Reaction Yield?
Reaction Yield – The amount of limiting reagent present at the start of a reaction determines the theoretical yield of the reaction, that is, the amount of product that would result if all the limiting reagents reacted. – The theoretical yield, then, is the maximum obtainable yield, predicted by the …
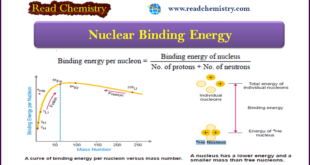
Read More »Nuclear Binding Energy: Definition, Formula, Explanation
– In this subject, we will discuss Nuclear Binding Energy: (Definition, Formula, Explanation) Nuclear Binding Energy – The atomic nucleus is made of protons and neutrons closely packed in a small volume. – Although there exist intensive repulsive forces between the component protons, the nucleus is not split apart. This …
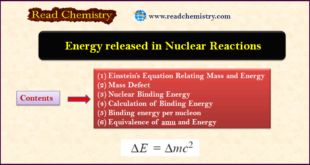
Read More »Energy released in Nuclear Reactions: Nuclear Binding Energy
– In this subject, we will discuss the energy released in Nuclear Reactions: Nuclear Binding Energy Einstein’s Equation Relating Mass and Energy – According to Albert Einstein, mass can be converted into energy and vice versa. – His famous equation relating mass and energy is: where E = energy ; m …
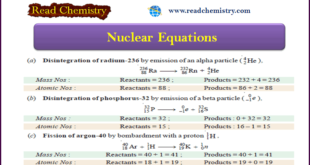
Read More »Nuclear Equations: Balancing, Rules, Practice
– In this subject, we will discuss the Nuclear Equations: Balancing, Rules, Practice Nuclear Equations – Similar to a chemical reaction, nuclear reactions can be represented by equations. – These equations involving the nuclei of the reactants and products are called nuclear equations. – The nuclear reactions occur by redistribution …
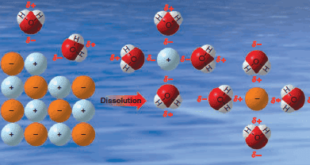
Read More »Solubility of Organic Compounds
Solubility of Organic Compounds – Intermolecular forces are of primary importance in explaining the solubility of substances. – Dissolution of a solid in a liquid is, in many respects, like the melting of a solid. – The orderly crystal structure of the solid is destroyed, and the result is the …
Read More »Intermolecular Forces in Organic compounds
– In this subject, we will discuss the Intermolecular Forces in Organic compounds Intermolecular Forces in Organic Compounds – The forces that act between molecules are not as strong as those between ions, but they account for the fact that even completely nonpolar molecules can exist in liquid and solid …
Read More »Limiting Reagent: Definition, Examples, Problems
– In this subject, we will discuss the Limiting Reagent (Definition, Examples, Problems) Limiting Reagent – When a chemist carries out a reaction, the reactants are usually not present in exact stoichiometric amounts, that is, in the proportions indicated by the balanced equation. – Because the goal of a reaction …
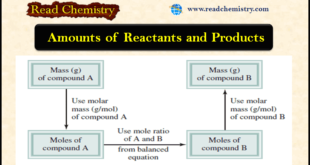
Read More »Amounts of Reactants and Products
– In this subject, we will discuss Amounts of Reactants and Products Amounts of Reactants and Products – A basic question raised in the chemical laboratory is (How much product will be formed from specific amounts of starting materials (reactants)?) Or in some cases, we might ask the reverse question: …
Read More »Physical Properties and Molecular Structure of Organic compound
** So far, we have said little about one of the most obvious characteristics of organic compounds— that is, their physical state or phase. Whether a particular substance is a solid, or a liquid, or a gas would certainly be one of the first observations that we would …
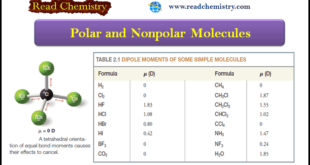
Read More »Polar and Nonpolar Molecules
– In this subject, we will discuss the Polar and Nonpolar Molecules. Dipole moment – The dipole moment is a physical property that can be measured experimentally. – It is defined as the product of the magnitude of the charge in electrostatic units (esu) and the distance that separates them …
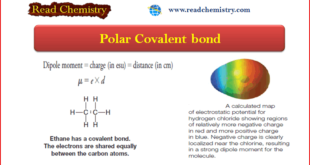
Read More »Polar Covalent Bond and Dipole moment
– In this subject, we will discuss the Polar Covalent Bond and Dipole moment. Polar Covalent Bonds – Covalent bonds form by sharing of electrons between atoms of similar electronegativities to achieve the configuration of a noble gas. – If we have a compound such as LiF in which the …
Read More »Nuclear Reaction: Definition, Types, Examples, Equations
– In this subject, we will discuss the Nuclear Reaction: Definition, Types, Examples, and Equations. Nuclear Reaction – In a chemical reaction, there is merely a rearrangement of extranuclear electrons. – The atomic nucleus remains intact. – A nuclear reaction involves a change in the composition of the nucleus. – …
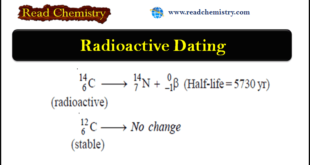
Read More »Radioactive Dating: Definition, Formula, Examples
– In this subject, we will discuss the Radioactive Dating: Definition, Formula, Examples Radioactivity – Several elements such as uranium and radium are unstable. – Their atomic nucleus breaks off its own accord to form a smaller atomic nucleus of another element. – The protons and neutrons in the unstable …
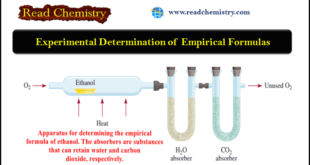
Read More »Experimental Determination of Empirical Formulas
Experimental Determination of Empirical Formulas ** The fact that we can determine the empirical formula of a compound if we know the percent composition enables us to identify compounds experimentally. ** The procedure is as follows. (1) Chemical analysis tells us the number of grams of each element …
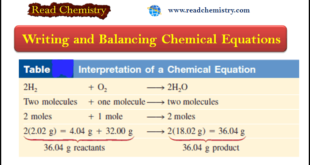
Read More »Chemical Equations – Writing and Balancing Chemical Equations
– In this subject, we will discuss Writing and Balancing Chemical Equations. Chemical Reactions and Chemical Equations – A chemical reaction is a process in which a substance (or substances) is changed into one or more new substances. – To communicate with one another about chemical reactions, chemists have devised …
Read More » Read Chemistry
Read Chemistry