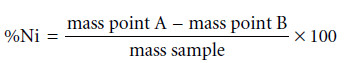What is Analytical Chemistry?
What is Analytical Chemistry?
Analytical chemistry is what analytical chemists do.
– Analytical chemistry is too broad and active a discipline for us to easily or completely define in an introductory textbook. Instead, we will try to say a little about what analytical chemistry is, as well as a little about what analytical chemistry is not.
– Analytical chemistry is often described as the area of chemistry responsible for characterizing the composition of matter, both qualitatively (what is present) and quantitatively (how much is present). This description is misleading.
– After all, almost all chemists routinely make qualitative or quantitative measurements.
– The argument has been made that analytical chemistry is not a separate branch of chemistry, but simply the application of chemical knowledge.
– In fact, you probably have performed quantitative and qualitative analyses in other chemistry courses.
– For example, many introductory courses in chemistry include qualitative schemes for identifying inorganic ions and quantitative analyses involving titrations.
– Unfortunately, this description ignores the unique perspective that analytical chemists bring to the study of chemistry.
The First distinction between analytical Chemistry and Chemical Analysis
– The craft of analytical chemistry is not in performing a routine analysis on a routine sample (which is more appropriately called chemical analysis), but in improving established methods, extending existing methods to new types of samples, and developing new methods for measuring chemical phenomena.
– Here’s one example of this distinction between analytical chemistry and chemical analysis.
– Mining engineers evaluate the economic feasibility of extracting an ore by comparing the cost of removing the ore with the value of its contents. To estimate its value they analyze a sample of the ore.
– The challenge of developing and validating the method providing this information is the analytical chemist’s responsibility.
– Once developed, the routine, daily application of the method becomes the job of the chemical analyst.
Another distinction between Analytical Chemistry and Chemical Analysis
– Another distinction between analytical chemistry and chemical analysis is that analytical chemists work to improve established methods.
– For example, several factors complicate the quantitative analysis of Ni2+ in ores, including the presence of a complex heterogeneous mixture of silicates and oxides, the low concentration of Ni2+ in ores, and the presence of other metals that may interfere in the analysis.
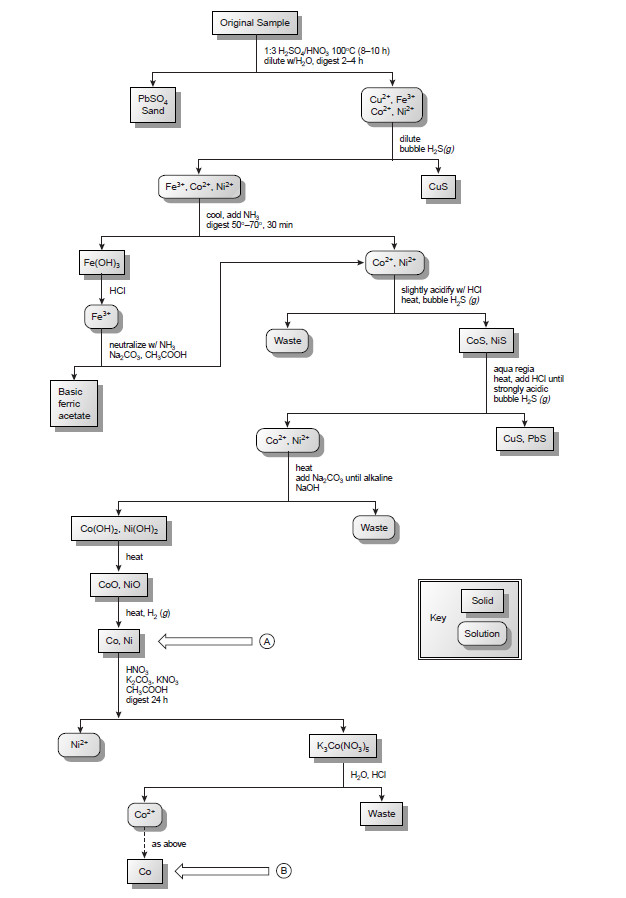
– The following Figure is a schematic outline of one standard method in use during the late nineteenth century.
– After dissolving a sample of the ore in a mixture of H2SO4 and HNO3, trace metals that interfere with the analysis, such as Pb2+, Cu2+ and Fe3+, are removed by precipitation.
– Any cobalt and nickel in the sampleare reduced to Co and Ni, isolated by filtration and weighed (point A).
– After dissolving the mixed solid, Co is isolated and weighed (point B).
– The amount of nickel in the ore sample is determined from the difference in the masses at points A and B.
– The combination of determining the mass of Ni2+ by difference, coupled with the need for many reactions and filtrations makes this procedure both time-consuming and difficult to perform accurately.
– The development, in 1905, of dimethylgloxime (DMG), a reagent that selectively precipitates Ni2+ and Pd2+, led to an improved analytical method for determining Ni2+ in ores.
– As shown in Figure, the mass of Ni2+ is measured directly, requiring fewer manipulations and less time.
– By the 1970s, the standard method for the analysis of Ni2+ in ores progressed from precipitating Ni(DMG)2 to flame atomic absorption spectrophotometry, resulting in an even more rapid analysis.
– Current interest is directed toward using inductively coupled plasmas for determining trace metals in ores.
Summary
– In summary, a more appropriate description of analytical chemistry is “. . . the science of inventing and applying the concepts, principles, and . . . strategies for measuring the characteristics of chemical systems and species.”
– Analytical chemists typically operate at the extreme edges of analysis, extending and improving the ability of all chemists to make meaningful measurements on smaller samples, on more complex samples, on shorter time scales, and on species present at lower concentrations.
– Throughout its history, analytical chemistry has provided many of the tools and methods necessary for research in the other four traditional areas of chemistry, as well as fostering multidisciplinary research in, to name a few, medicinal chemistry, clinical chemistry, toxicology, forensic chemistry, material science, geochemistry, and environmental chemistry.
– You will come across numerous examples of qualitative and quantitative methods in this text, most of which are routine examples of chemical analysis.
– It is important to remember, however, that nonroutine problems prompted analytical chemists to develop these methods. Whenever possible, we will try to place these methods in their appropriate historical context.
– In addition, examples of current research problems in analytical chemistry are scattered throughout the text.
– The next time you are in the library, look through a recent issue of an analytically oriented journal, such as Analytical Chemistry.
– Focus on the titles and abstracts of the research articles. Although you will not recognize all the terms and methods, you will begin to answer for yourself the question “What is analytical chemistry”?