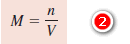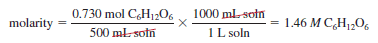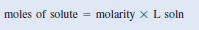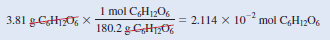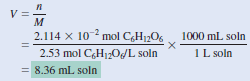Molarity: definition, formula, solved Problems
– In this subject, we will discuss the Molarity: definition, formula, solved Problems
– To study solution stoichiometry, we must know how much of the reactants are present in a solution and also how to control the amounts of reactants used to bring about a reaction in aqueous solution.
The concentration of a solution
– The concentration of a solution is the amount of solute present in a given amount of solvent, or a given amount of solution.
(For this discussion, we will assume the solute is a liquid or a solid and the solvent is a liquid.)
– The concentration of a solution can be expressed in many different ways.
– Here we will consider one of the most commonly used units in chemistry, molarity (M), or molar concentration.
Molarity (M)
– Molarity is The number of moles of solute per liter of solution.
– where (n) denotes the number of moles of solute and (V) is the volume of the solution in liters.
– A 1.46 molar glucose (C6H12O6 ) solution, written as 1.46 M (C6H12O6 ), contains 1.46 moles of the solute (C6H12O6) in 1 L of the solution.
– Of course, we do not always work with solution volumes of 1 L.
– Thus, a 500-mL solution containing 0.730 mole of (C6H12O6) also has a concentration of 1.46 M:
– Note that concentration, like density, is an intensive property, so its value does not depend on how much of the solution is present.
– It is important to keep in mind that molarity refers only to the amount of solute originally dissolved in water and does not take into account any subsequent processes, such as the dissociation of a salt or the ionization of an acid.
– Consider what happens when a sample of potassium chloride (KCl) is dissolved in enough water to make a 1 M solution:
– Because KCl is a strong electrolyte, it undergoes complete dissociation in solution.
– Thus, a 1 M KCl solution contains 1 mole of K+ ions and 1 mole of Cl–ions, and no KCl units are present.
– The concentrations of the ions can be expressed as [K+] = 1 M and [Cl– ] = 1 M, where the square brackets [ ] indicate that the concentration is expressed in molarity.
– Similarly, in a 1 M barium nitrate [Ba(NO3)2] solution:
– we have [Ba2+] = 1 M and [NO3– ] = 2 M and no Ba(NO3)2units at all.
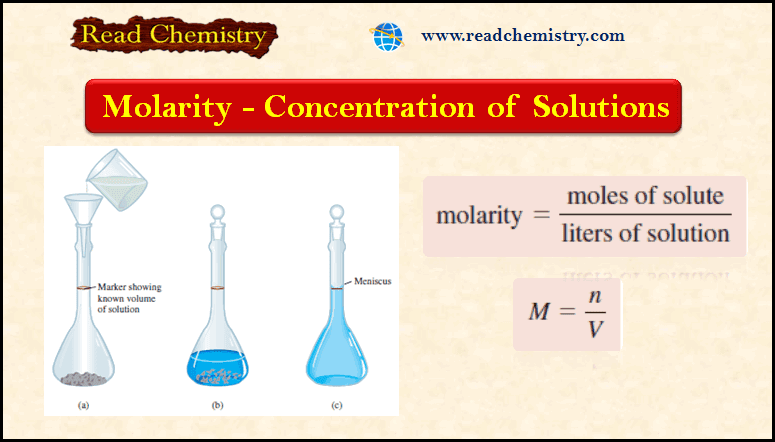
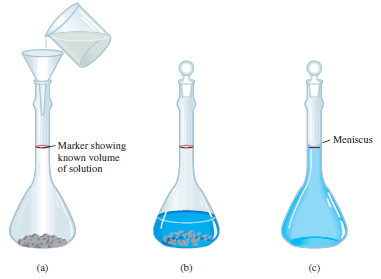
Preparing a solution of known Molarity
– The procedure for preparing a solution of known molarity is as follows
(1) The solute is accurately weighed and transferred to a volumetric flask through a funnel.
(2) Water is added to the flask, which is carefully swirled to dissolve the solid.
(3) After all the solid has dissolved, more water is added slowly to bring the level of solution exactly to the volume mark.
– Knowing the volume of the solution in the flask and the quantity of compound (the number of moles) dissolved, we can calculate the molarity of the solution using Equation (1).
– Note that this procedure does not require knowing the amount of water added, as long as the volume of the final solution is known.
Solved Problems on Molarity
Example (1): How many grams of potassium dichromate (K2Cr2 O7) are required to prepare a 250 mL solution whose concentration is 2.16 M?
Strategy
– How many moles of K2Cr2O7 does a 1-L (or 1000 mL) 2.16 M K2Cr2O7 solution contain? A 250-mL solution? How would you convert moles to grams?
Solution
– The first step is to determine the number of moles of K2Cr2O7 in 250 mL or 0.250 L of a 2.16 M solution.
– Rearranging Equation (1) gives:
– The molar mass of K2Cr2O7 is 294.2 g, so we write:
Check
– As a ball-park estimate, the mass should be given by:
[molarity (mol/L) × volume (L) × molar mass (g/mol)] =
[2 mol/L × 0.25 L × 300 g/mol] = 150 g.
– So the answer is reasonable.
Example (2): In a biochemical assay, a chemist needs to add 3.81 g of glucose to a reaction mixture. Calculate the volume in milliliters of a 2.53 M glucose solution she should use for the addition.
Strategy
– We must first determine the number of moles contained in 3.81 g of glucose and then use Equation (2) to calculate the volume.
Solution
– From the molar mass of glucose, we write:
– Next, we calculate the volume of the solution that contains 2.114 × 10-2 mole of the solute.
– Rearranging Equation (2) gives:
Check
– One liter of the solution contains 2.53 moles of C6H12O6 .
– Therefore, the number of moles in 8.36 mL or 8.36 10-3 L is (2.53 mol × 8.36 × 10-3) or 2.12 × 10-2 mol.
– The small difference is due to the different ways of rounding off.
- Reference: Chemistry / Raymond Chang, Williams College /(10th edition).