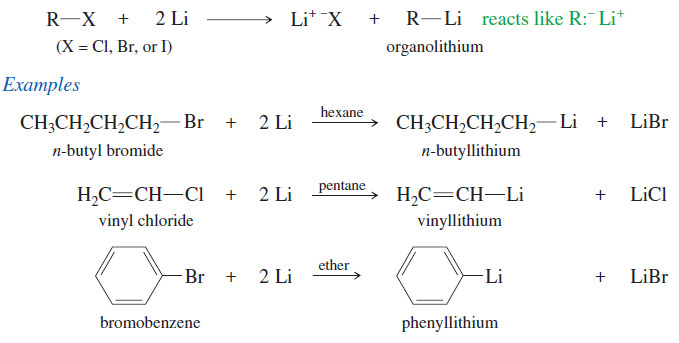
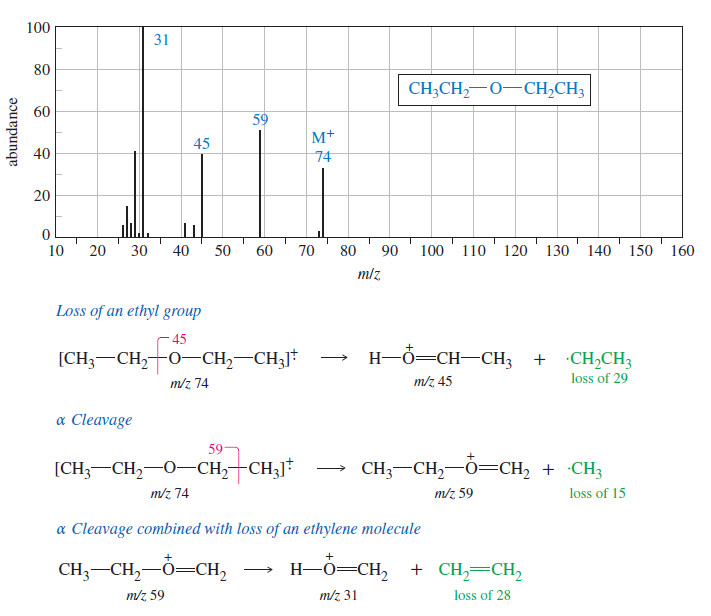
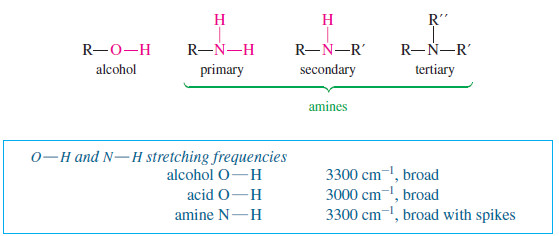
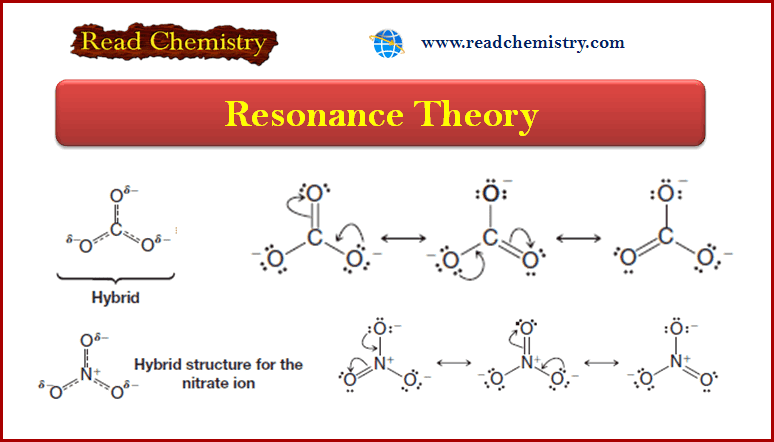
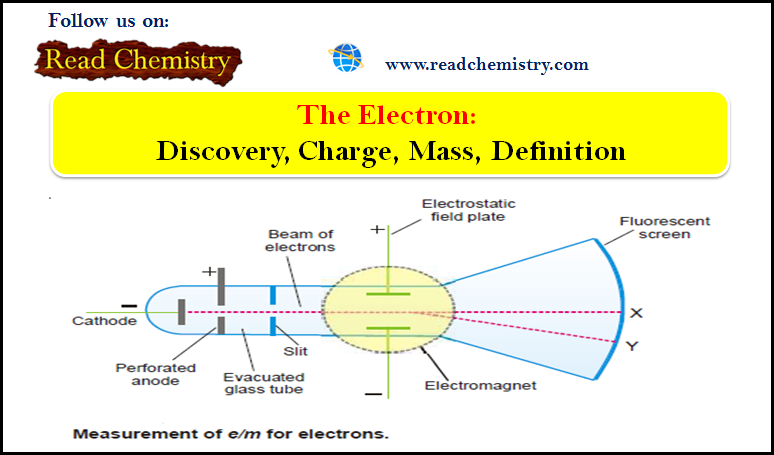
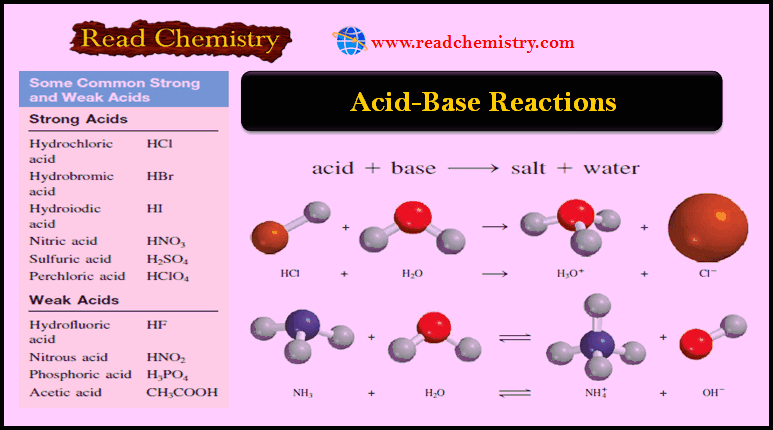
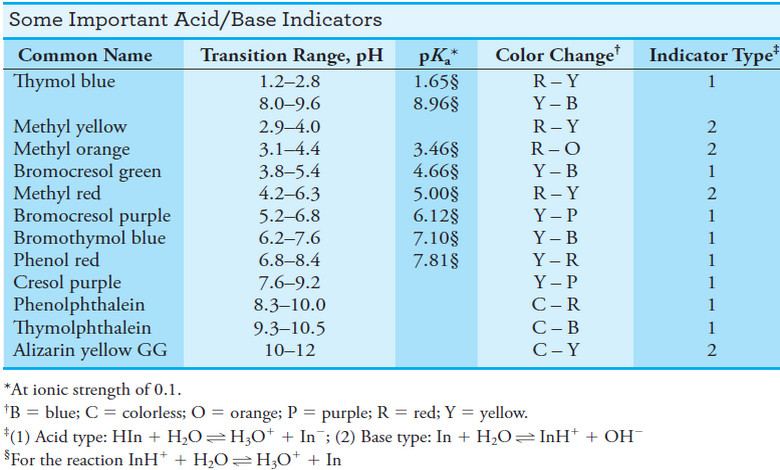
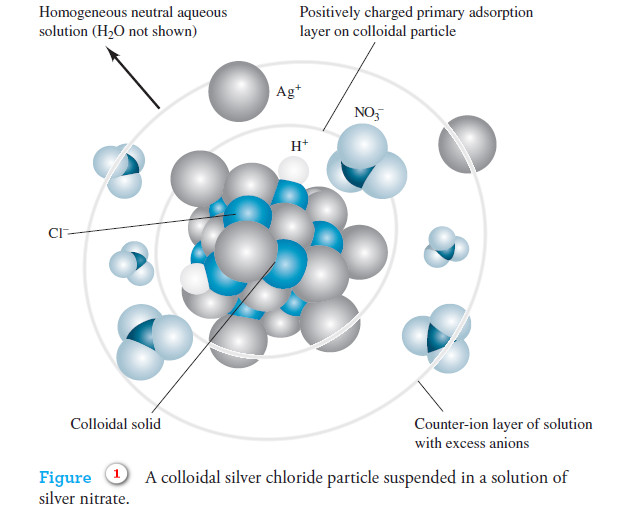
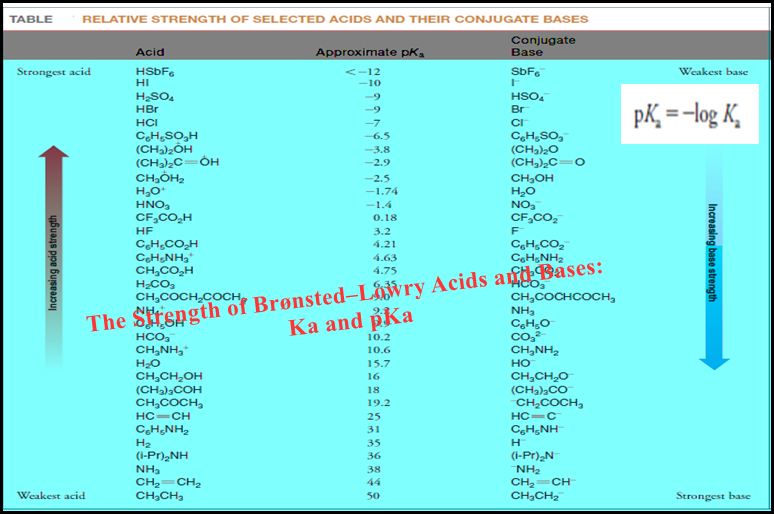
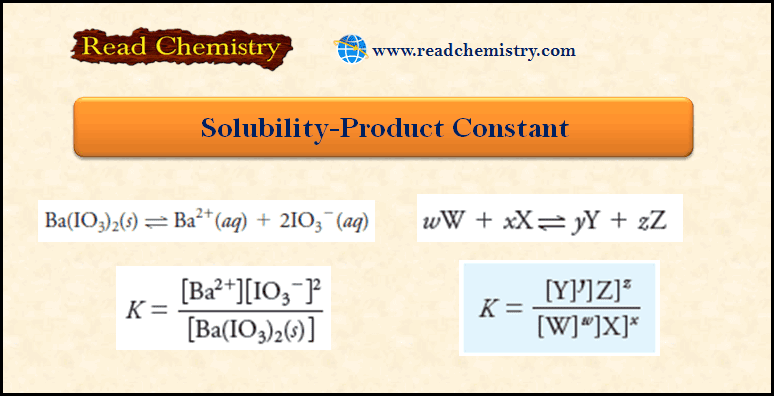
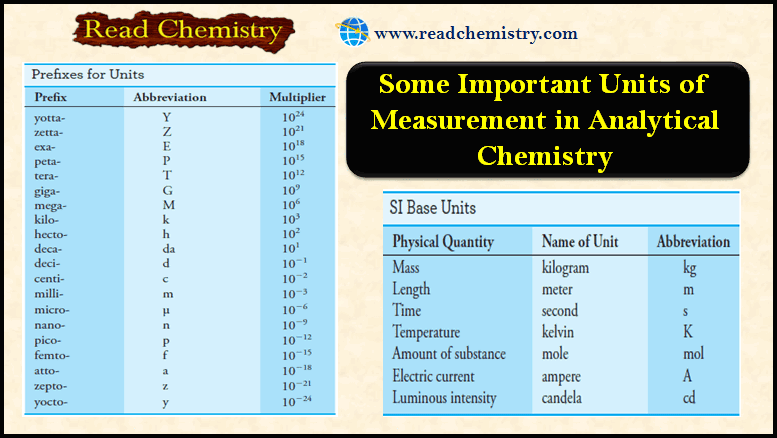
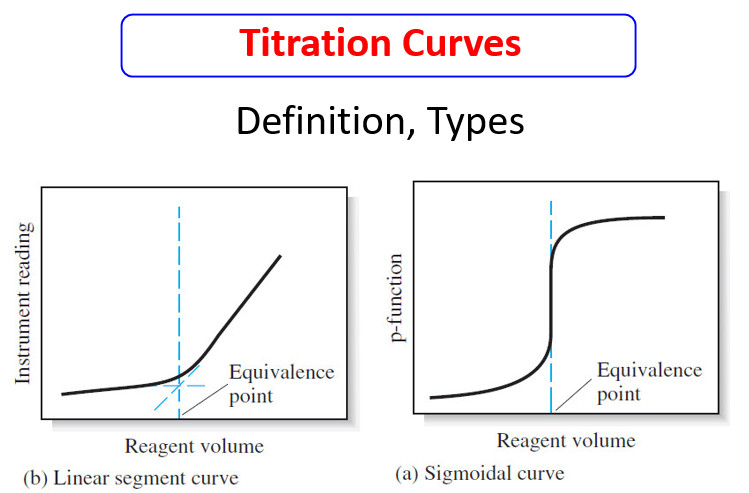
Popular Posts
-
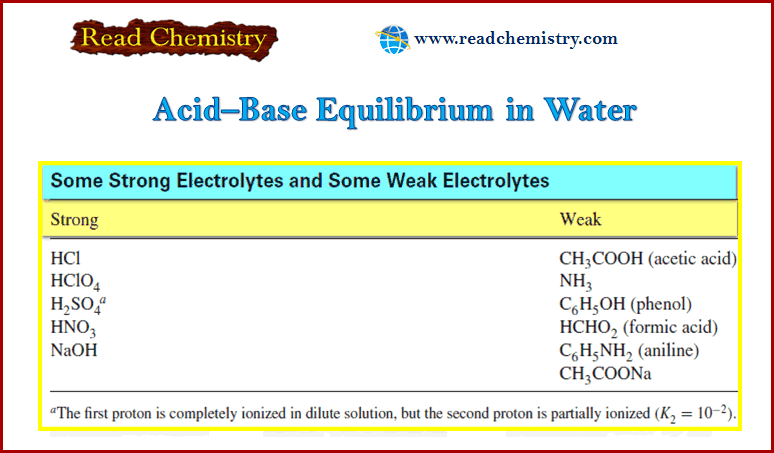
Analytical Chemistry
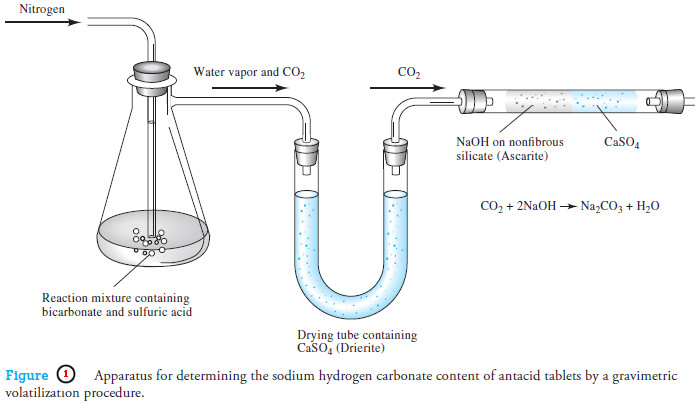
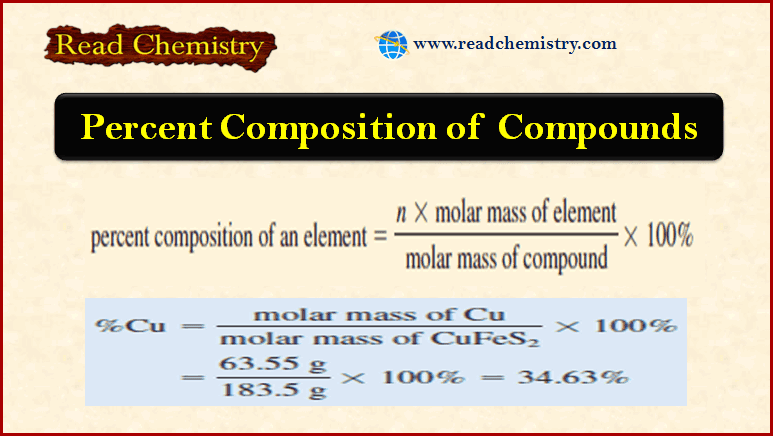
Applications of Gravimetric methods
Applications of Gravimetric methods – Gravimetric methods have been developed for most inorganic anions and cations, as well as for…
Read More » -
Free book
Concise Physical Chemistry book by Donald W.Rogers
– In this subject, we will discuss the free download of Concise Physical Chemistry book by Donald W.Rogers The Preface…
Read More » -
General Chemistry
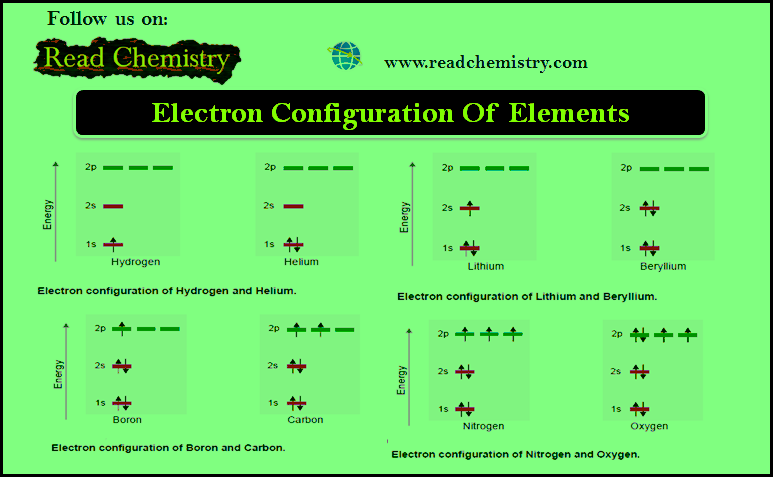
Electron Configuration Of Elements
– In this subject, we will discuss the rules of Electron Configuration Of Elements Electron Configuration Of Elements – We…
Read More » -
Organic Chemistry
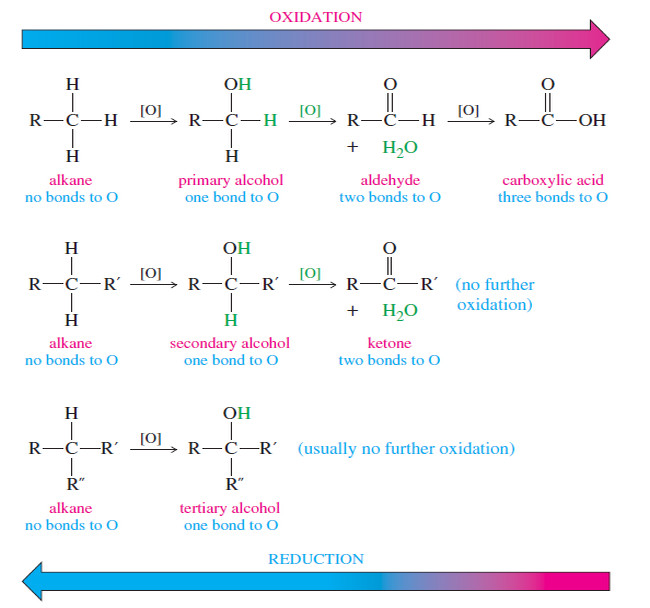
Oxidation states of Alcohols and Related Functional Groups
Oxidation states of Alcohols and Related Functional Groups – Oxidation states of Alcohols leads to ketones, aldehydes, and carboxylic acids.…
Read More » -
Organic Chemistry
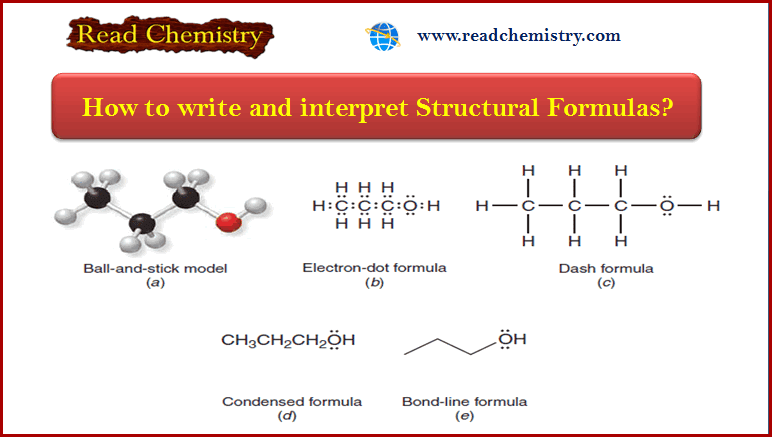
How to write and interpret Structural Formulas?
** Organic chemists use a variety of formats to write structural formulas. ** Structural Formulas are written…
Read More » -
Physical Chemistry
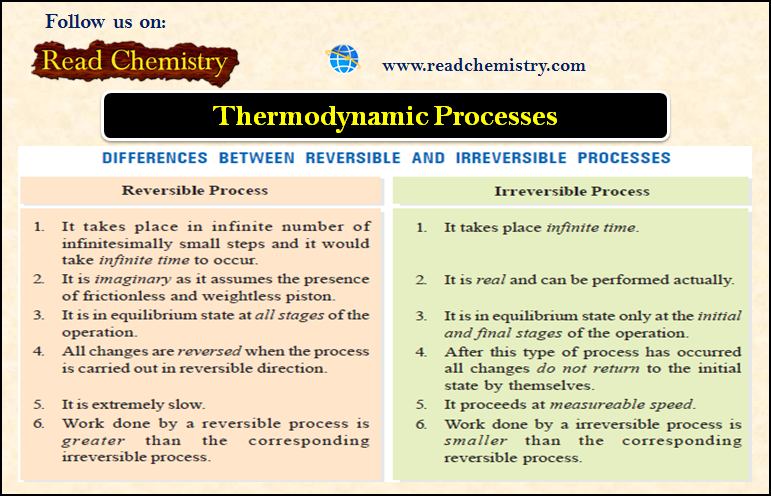
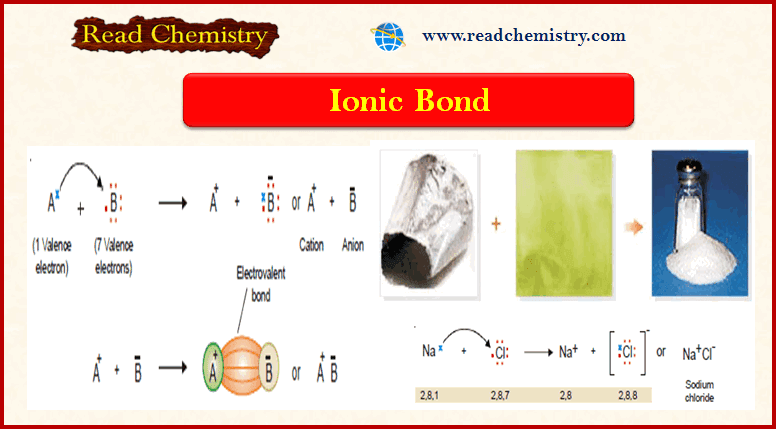
Thermodynamic Processes
– Thermodynamic Processes involve the change of conditions (temperature, pressure, and volume). Thermodynamic Processes – When a thermodynamic system changes…
Read More »
-
Organic Chemistry
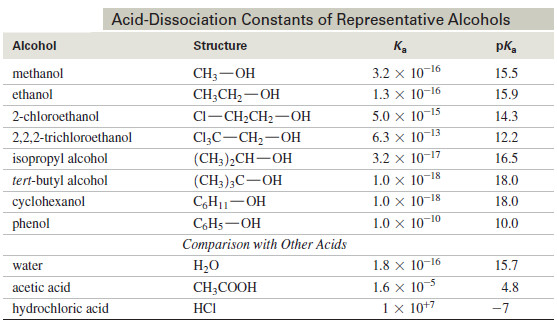
Acidity of Alcohols and Phenols
Acidity of Alcohols and Phenols – we will talk here about some Acidity of Alcohols…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
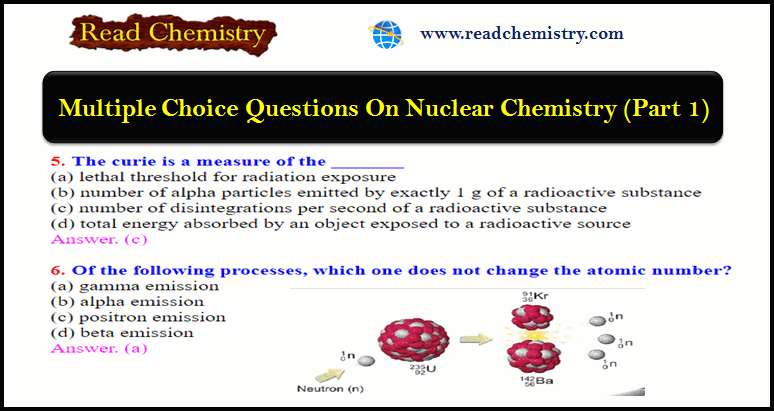
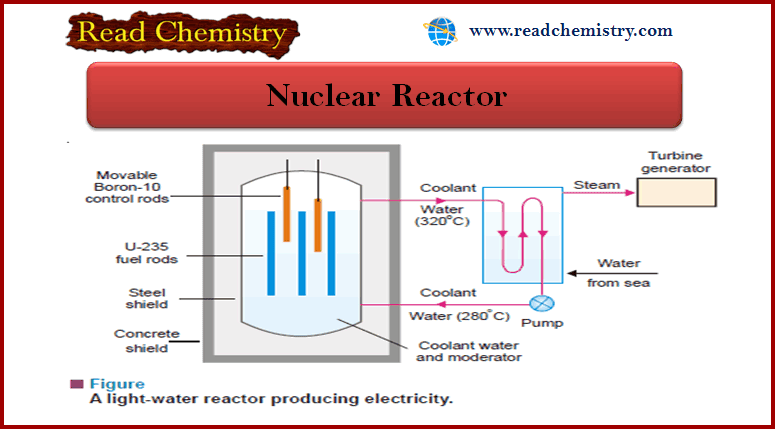
Nuclear Chemistry Quiz: Questions and Answers
Nuclear Chemistry Quiz – In this subject, you will find 40 questions and answers MCQ…
Read More » -
-
-
-
-
-
-
-
-
-
-
Free book
Fundamentals of Chemistry book by Romain Elsair – Free download
– In this subject, we will discuss the free download of Fundamentals of Chemistry book…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
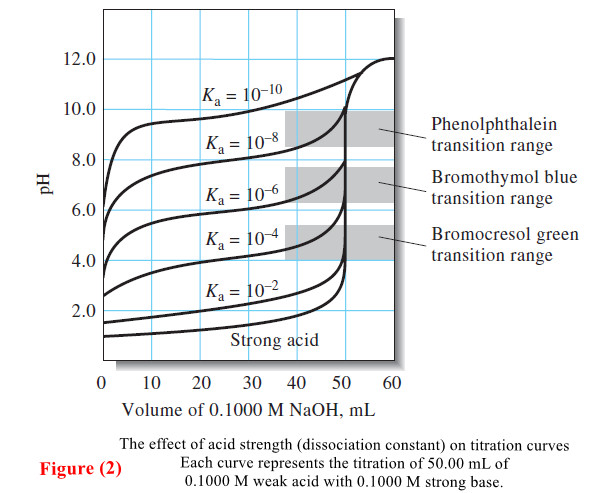
Indicators and Solutions for acid-base titration
– In this topic, we will discuss the Indicators and Solutions for acid-base titration. Indicators…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
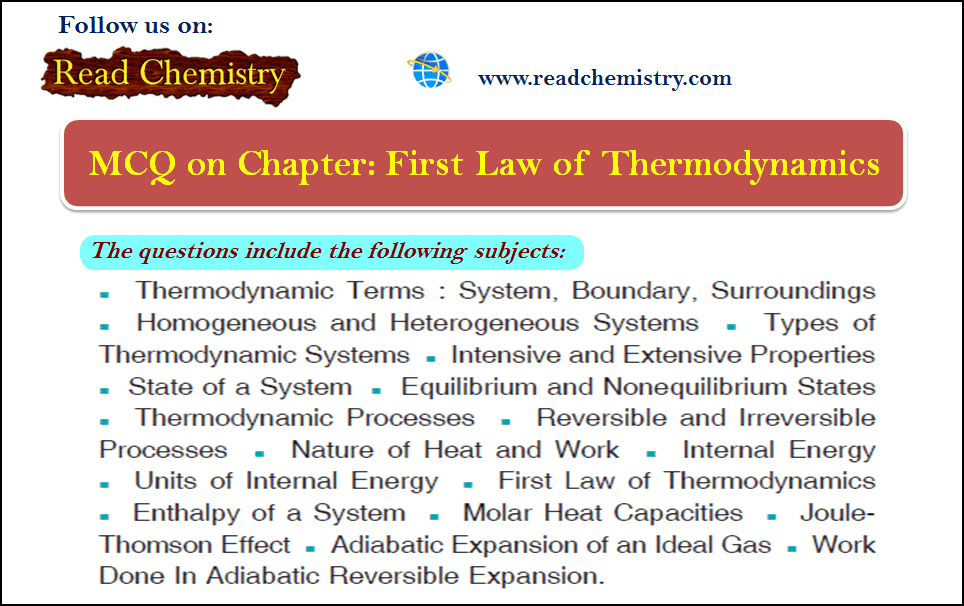
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-