Analytical Chemistry involves techniques and methods to identify, quantify, and understand chemical substances. It supports quality control, research, environmental analysis, and forensic science, ensuring accuracy and precision in measurements.
Analytical Chemistry
-
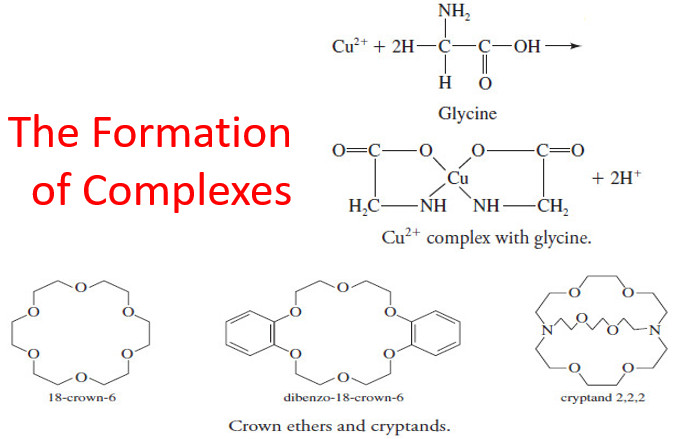
The Formation of Complexes
The Formation of Complexes – Most metal ions react with electron-pair donors to form coordination compounds or complexes. – The…
Read More » -
Applications of Neutralization Titrations
– In this topic, we will discuss The Applications of Neutralization Titrations. Typical Applications of Neutralization Titrations – Neutralization titrations…
Read More » -
Reagents for Neutralization Titrations
– In this subject, we will discuss the Reagents for Neutralization Titrations. Reagents for Neutralization Titrations – we noted before…
Read More » -
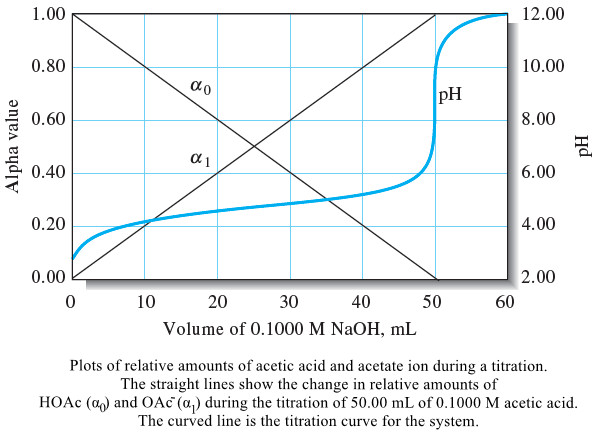
The Composition of Solutions During acid/Base Titration
– In this topic, we will discuss The Composition of Solutions During acid/Base Titration. The Composition of Solutions During acid/Base…
Read More » -
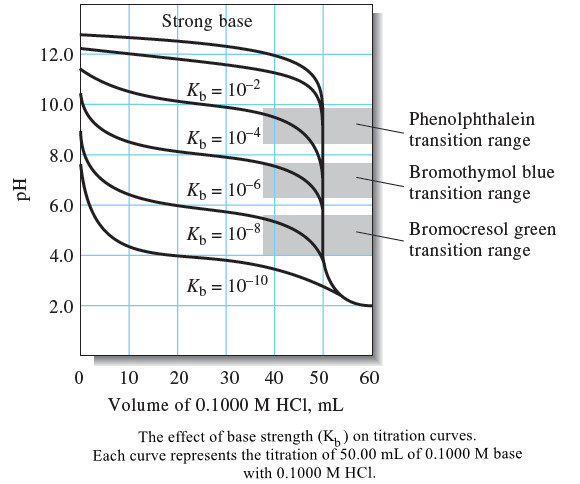
Titration Curves for Weak Bases
– In this topic, we will discuss The titration Curves for Weak Bases. Titration Curves for Weak Bases – The…
Read More » -
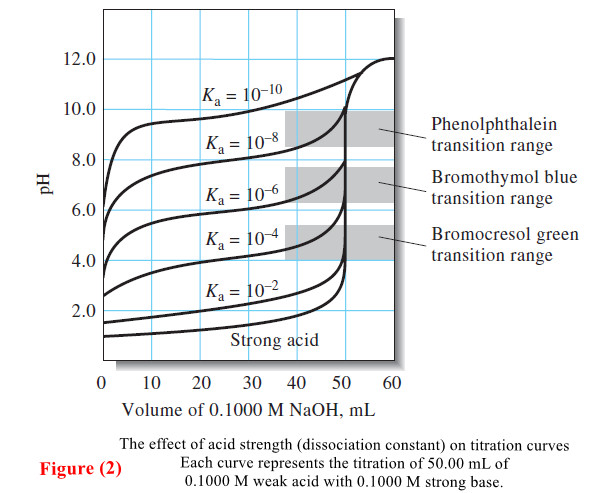
Titration Curves for Weak Acids
Titration Curves for Weak Acids – Four distinctly different types of calculations are needed to compute values for a weak…
Read More » -
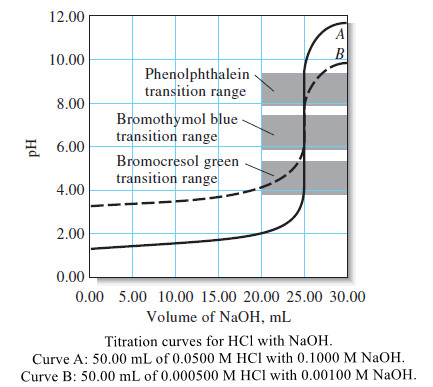
Titration of Strong Acids and Bases
– In this topic, we will discuss The Titration of Strong Acids and Bases. Introduction to Titration of Strong Acids…
Read More » -
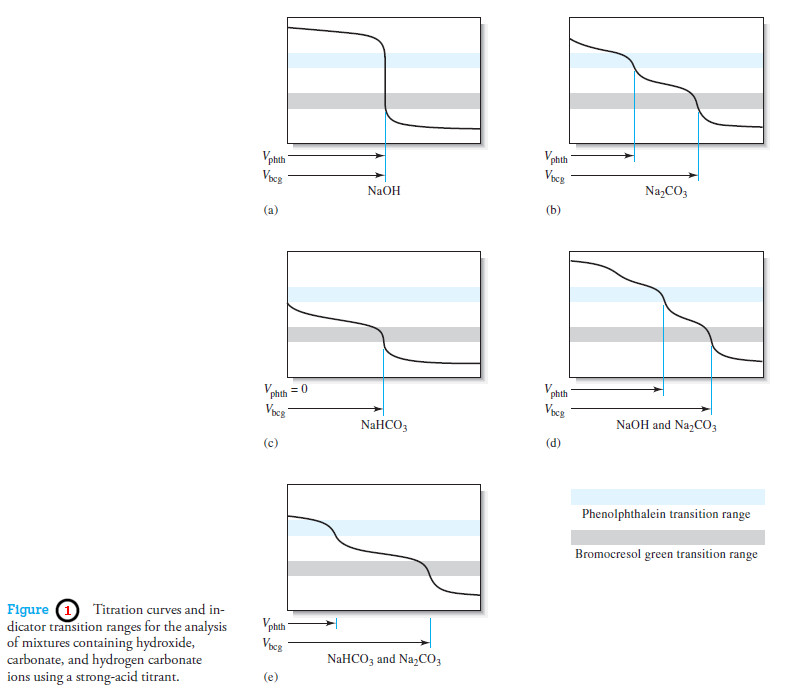
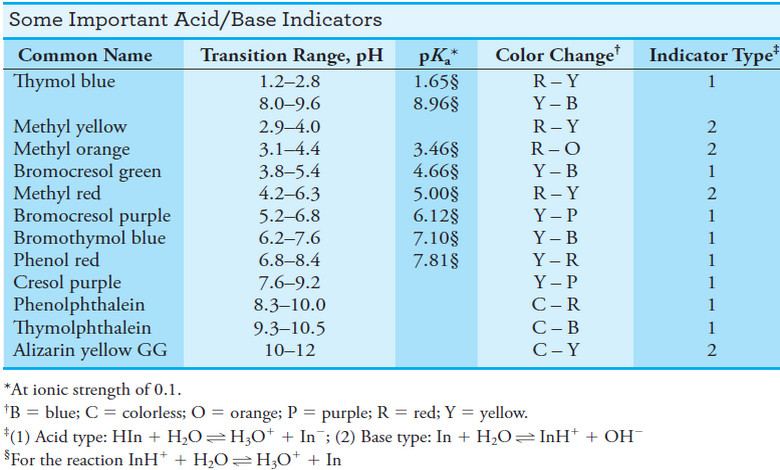
Indicators and Solutions for acid-base titration
– In this topic, we will discuss the Indicators and Solutions for acid-base titration. Indicators and Solutions for acid-base titration…
Read More » -
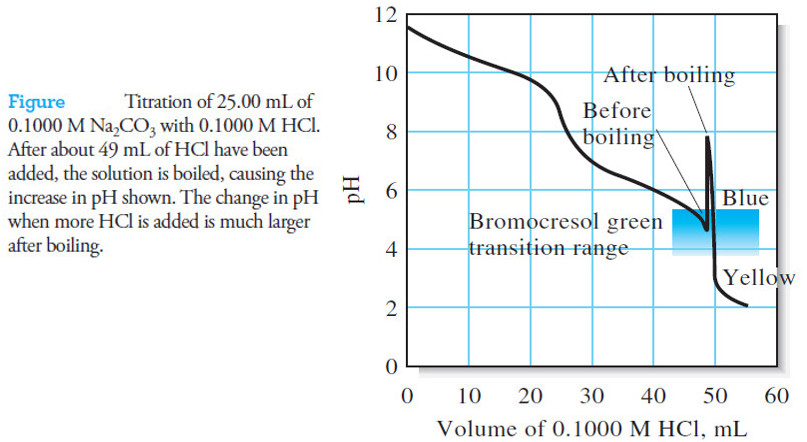
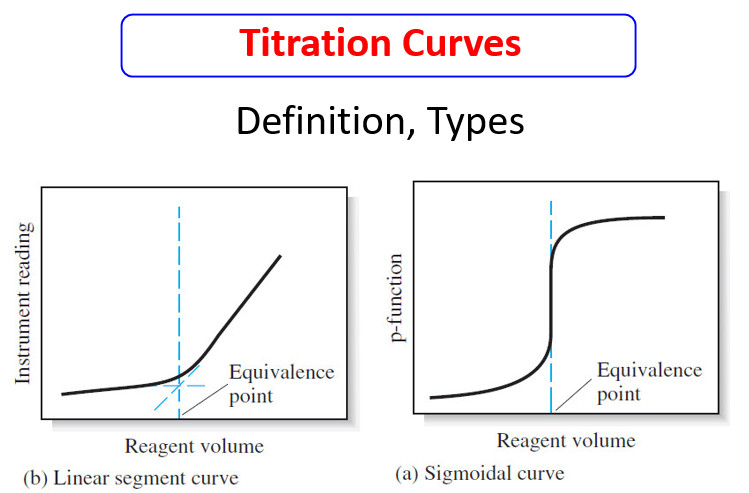
Titration Curves in Analytical Chemistry : Definition, Types
– In this topic, we will discuss the Titration Curves in Analytical Chemistry : Definition and Types Titration Curves –…
Read More » -
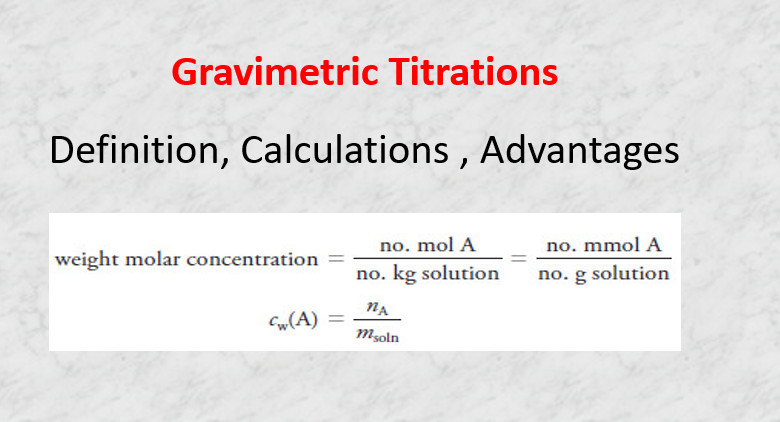
Gravimetric Titrations | Definition, Calculations & Advantages
Gravimetric titrations – Mass (weight) or gravimetric titrations differ from their volumetric counterparts in that the mass of titrant is…
Read More » -
Some Terms Used in Volumetric Titration
Some Terms Used in Volumetric Titration – A standard solution (or a standard titrant) is a reagent of known concentration…
Read More » -
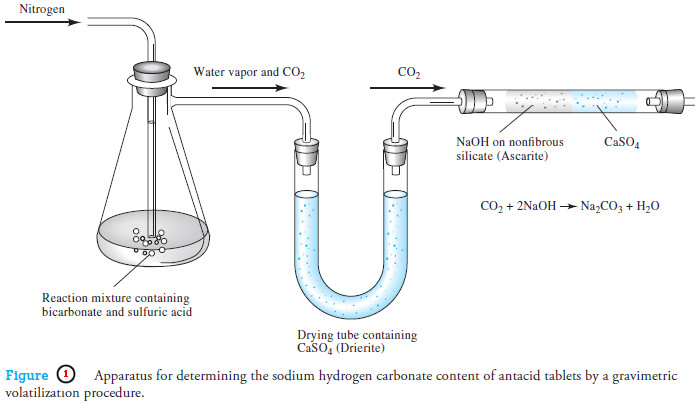
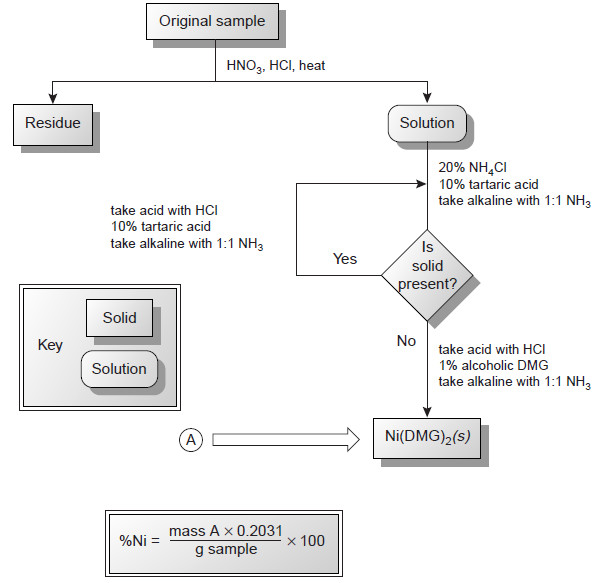
Applications of Gravimetric methods
Applications of Gravimetric methods – Gravimetric methods have been developed for most inorganic anions and cations, as well as for…
Read More » -
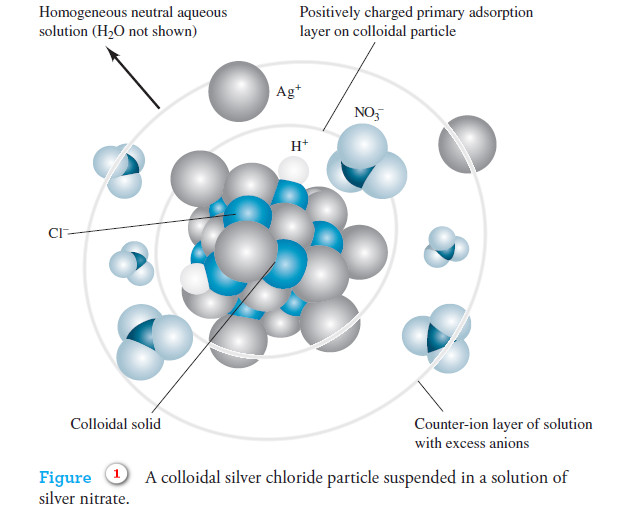
Precipitation Gravimetry
– In this topic, we will discuss the Precipitation Gravimetry as an important one of Gravimetric Analysis in analytical chemistry.…
Read More » -
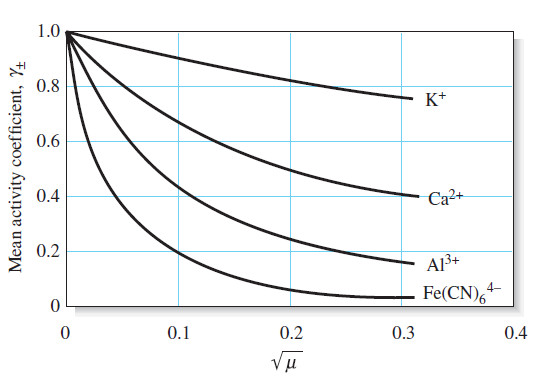
Activity Coefficients : Definition, Equation, Examples, Properties
– In this topic, we will discuss Activity coefficients : Definition, Equation, Examples and Properties. Activity Coefficients – Chemists use…
Read More » -
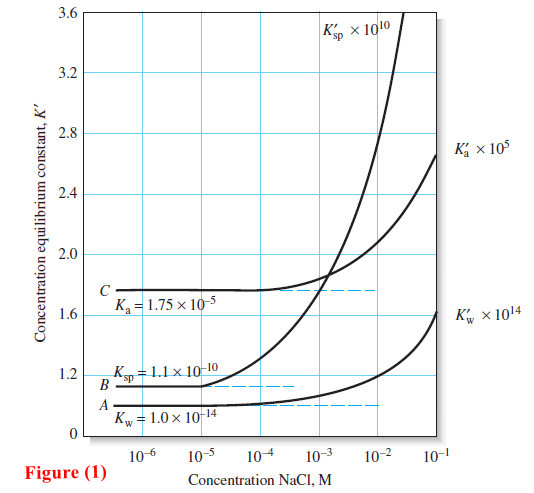
The Effect of Electrolyte on Chemical Equilibria
The Effect of Electrolyte on Chemical Equilibria – Experimentally, we find that the position of most solution equilibria depends on…
Read More » -
What is Analytical Chemistry?
What is Analytical Chemistry? Analytical chemistry is what analytical chemists do. – Analytical chemistry is too broad and active a…
Read More » -
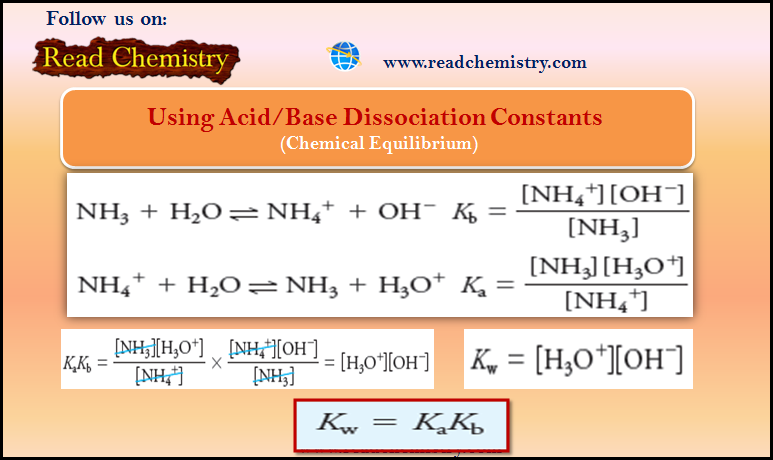
Acid and Base Dissociation Constants (Ka and Kb)
– In this subject, we will discuss the Acid and Base Dissociation Constants (Ka and Kb) Acid and Base Dissociation…
Read More » -
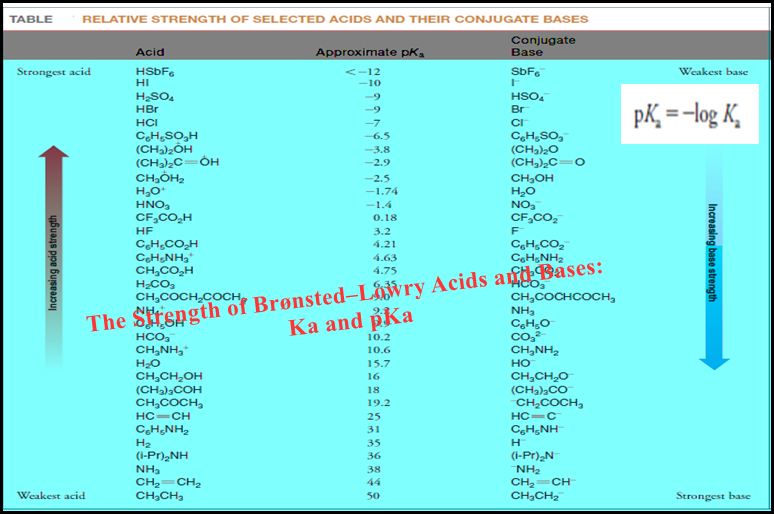
Bronsted-Lowry Acid Strength: Ka and pKa
– In this subject, we will discuss the Bronsted-Lowry Acid Strength: Ka and pKa Bronsted-Lowry Acid Strength: Ka and pKa…
Read More » -
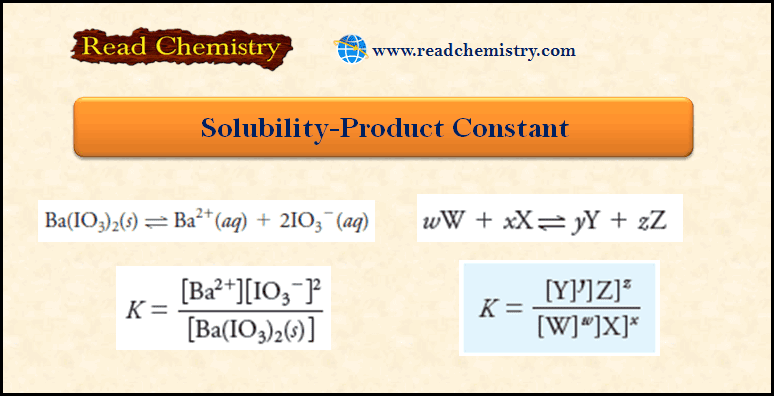
Solubility Product Ksp: Definition, Formula, Problems
– In this subject, we will discuss the Solubility Product (Ksp): Definition, Formula, Problems Solubility Product Constant Ksp – Most,…
Read More » -
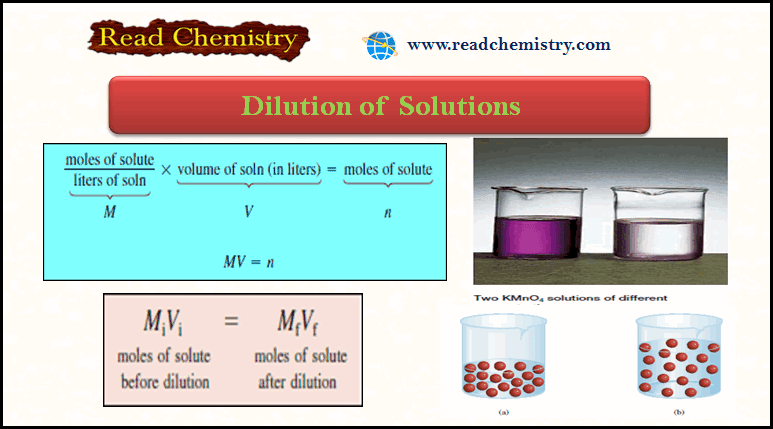
Dilution of Solutions
– In this subject, we will discuss the Dilution of Solutions and Dilution Law. Dilution of Solutions – Concentrated solutions…
Read More »