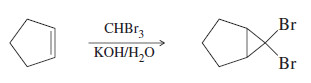Addition of Carbenes to Alkenes
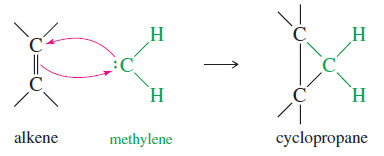
– Methylene (:CH2) is the simplest of the carbenes: uncharged, reactive intermediates that have a carbon atom with two bonds and two nonbonding electrons.
– Like borane (BH3), methylene is a potent electrophile because it has an unfilled octet.
– It adds to the electronrich pi bond of an alkene to form a cyclopropane.
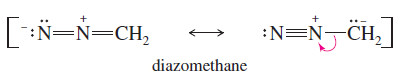
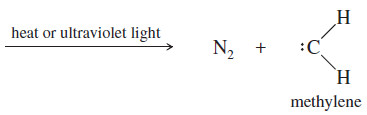
– Heating or photolysis of diazomethane gives nitrogen gas and methylene:
– The methylene generated from diazomethane reacts with alkenes to form cyclopropanes, but diazomethane is very toxic and explosive, and the methylene generated is so reactive that it forms many side products.
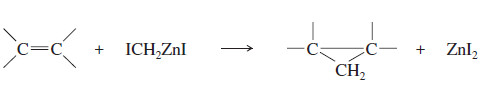
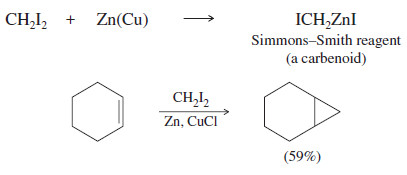
– A safer and more reliable way to make cyclopropanes is with the Simmons Smith reagent.
(A) The Simmons–Smith Reaction
– The Simmons–Smith reagent, named for the two DuPont chemists who discovered it, is made by adding methylene iodide to the “zinc–copper couple” (zinc dust that has been activated with an impurity of copper).
– The reagent probably resembles iodomethyl zinc iodide, ICH2ZnI.
– This kind of reagent is called a carbenoid because it reacts much like a carbene, but it does not actually contain a divalent carbon atom.
(B) Formation of Carbenes by Alpha Elimination
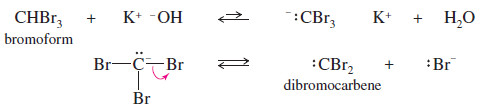
– Carbenes are also formed by reactions of halogenated compounds with bases.
– If a carbon atom has bonds to at least one hydrogen and to enough halogen atoms to make the hydrogen slightly acidic, it may be possible to form a carbene.
– For example, bromoform (CHBr3) reacts with a 50% aqueous solution of potassium hydroxide to form dibromocarbene.
– This dehydrohalogenation is called an alpha elimination (α elimination) because the hydrogen and the halogen are lost from the same carbon atom.
– The more common dehydrohalogenations (to form alkenes) are called beta eliminations (β elimination) because the hydrogen and the halogen are lost from adjacent carbon atoms.
– Dibromocarbene formed from CHBr3 can add to a double bond to form a dibromocyclopropane.
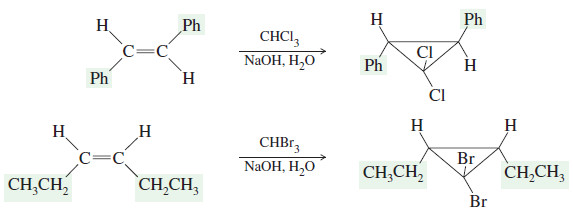
– The products of these cyclopropanations retain any cis or trans stereochemistry of the reactants
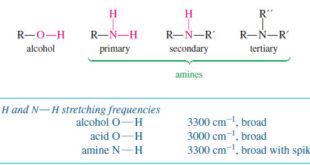
 Read Chemistry
Read Chemistry