Resolution of Enantiomers
Resolution of Enantiomers
– Pure enantiomers of optically active compounds are often obtained by isolation from biological sources.
– Most optically active molecules are found as only one enantiomer in living organisms.
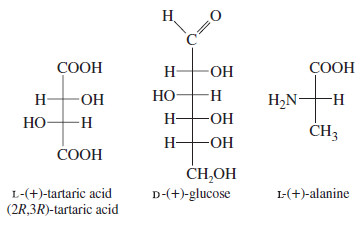
– For example, pure (+)-tartaric acid can be isolated from the precipitate formed by yeast during the fermentation of wine.
– Pure (+) glucose is obtained from many different sugar sources, such as grapes, sugar beets, sugarcane, and honey.
– Alanine is a common amino acid found in protein as the pure (+) enantiomer.
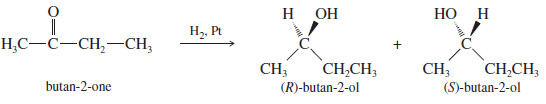
– When a chiral compound is synthesized from achiral reagents, however, a racemic mixture of enantiomers results.
– For example, we saw that the reduction of butan-2 one (achiral) to butan-2-ol (chiral) gives a racemic mixture
– If we need one pure enantiomer of butan-2-ol, we must find a way of separating it from the other enantiomer.
– The separation of enantiomers is called resolution, and it is a different process from the usual physical separations.
– A chiral probe is necessary for the resolution of enantiomers; such a chiral compound or apparatus is called a resolving agent.
– In 1848, Louis Pasteur noticed that a salt of racemic (±)-tartaric acid crystallizes into mirror-image crystals.
– Using a microscope and a pair of tweezers, he physically separated the enantiomeric crystals.
– He found that solutions made from the “left handed” crystals rotate polarized light in one direction and solutions made from the “right-handed” crystals rotate polarized light in the opposite direction.
– Pasteur had accomplished the first artificial resolution of enantiomers.
– Unfortunately, few racemic compounds crystallize as separate enantiomers, and other methods of separation are required.
Chemical Resolution of Enantiomers
– The traditional method for resolving a racemic mixture into its enantiomers is to use an enantiomerically pure natural product that bonds with the compound to be resolved.
– When the enantiomers of the racemic compound bond to the pure resolving agent, a pair of diastereomers results.
– The diastereomers are separated, then the resolving agent is cleaved from the separated enantiomers.
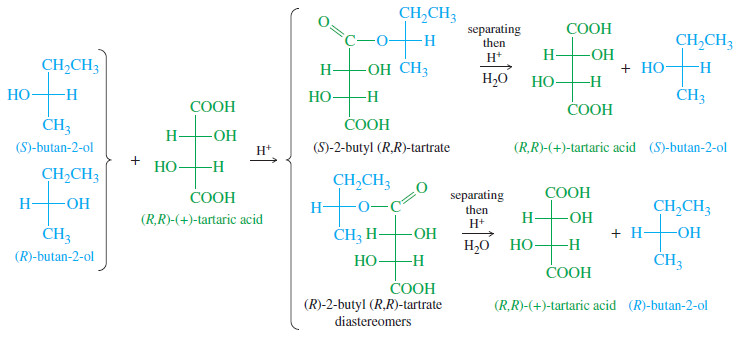
– Let’s consider how we might resolve a racemic mixture of (R)- and (S)-butan-2-ol.
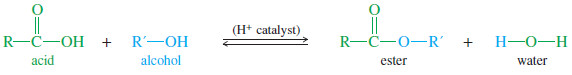
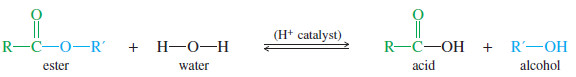
– We need a resolving agent that reacts with an alcohol and that is readily available in an enantiomerically pure state. A carboxylic acid combines with an alcohol to form an ester.
– Although we have not yet studied the chemistry of esters, the following equation shows how an acid and an alcohol can combine with the loss of water to form an ester.
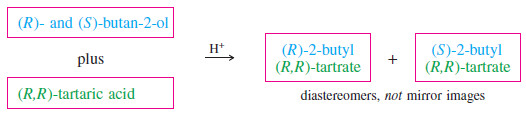
– For our resolving agent, we need an optically active chiral acid to react with butan-2-ol. Any winery can provide large amounts of pure (+)-tartaric acid.
– The following Figure shows that diastereomeric esters are formed when (R)- and (S)-butan-2-ol react with (+)-tartaric acid. We can represent the reaction schematically as follows:
– The diastereomers of 2-butyl tartrate have different physical properties, and they can be separated by conventional distillation, recrystallization, or chromatography.
– Separation of the diastereomers leaves us with two flasks, each containing one of the diastereomeric esters.
– The resolving agent is then cleaved from the separated enantiomers of butan-2-ol by the reverse of the reaction used to make the ester.
– Adding an acid catalyst and an excess of water to an ester drives the equilibrium toward the acid and the alcohol:
– Hydrolysis of (R)-butan-2-ol tartrate gives (R)-butan-2-ol and (+)-tartaric acid, and hydrolysis of (S)-butan-2-ol tartrate gives (S)-butan-2-ol and (+)-tartaric acid.
– The recovered tartaric acid would probably be thrown away, since it is cheap and nontoxic.
– Many other chiral resolving agents are expensive, so they must be carefully recovered and recycled
Chromatographic Resolution of Enantiomers
– Chromatography is a powerful method for separating compounds.
– One type of chromatography involves passing a solution through a column containing particles whose surface tends to adsorb organic compounds.
– Compounds that are adsorbed strongly spend more time on the stationary particles; they come off the column later than less strongly adsorbed compounds, which spend more time in the mobile solvent phase.
– In some cases, enantiomers may be resolved by passing the racemic mixture through a column containing particles whose surface is coated with chiral molecules (see Figure).
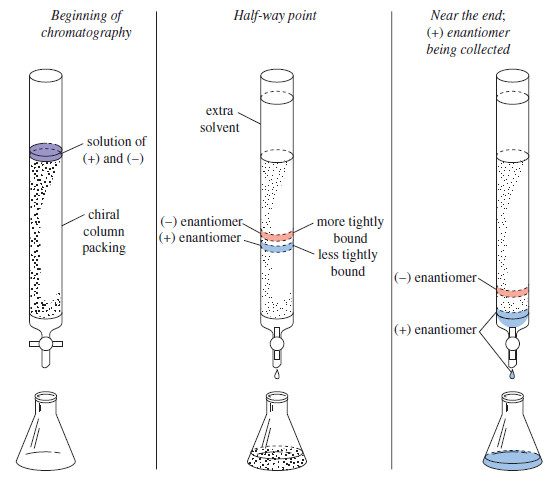
– As the solution passes through the column, the enantiomers form weak complexes, usually through hydrogen bonding, with the chiral column packing.
– The solvent flows continually through the column, and the dissolved enantiomers gradually move along, retarded by the time they spend complexed with the column packing.
– The special feature of this chromatography is the fact that the enantiomers form diastereomeric complexes with the chiral column packing.
– These diastereomeric complexes have different physical properties.
– They also have different binding energies and different equilibrium constants for complexation.
– One of the two enantiomers will spend more time complexed with the chiral column packing.
– The more strongly complexed enantiomer passes through the column more slowly and emerges from the column after the faster-moving (more weakly complexed) enantiomer.