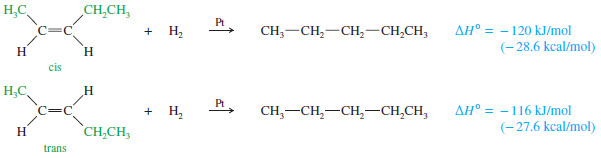Stability of Alkenes
Stability of Alkenes
– In making alkenes, we often find that the major product is the most stable alkene.
– Many reactions also provide opportunities for double bonds to rearrange to more stable isomers.
– Therefore, we need to know how the stability of an alkene depends on its structure.
– Stabilities can be compared by converting different compounds to a common product and comparing the amounts of heat given off.
– One possibility would be to measure heats of combustion from converting alkenes to CO2 and H2O.
– Heats of combustion are large numbers (thousands of kJ per mole), and measuring small differences in these large numbers is difficult.
– Instead, alkene energies are often compared by measuring the heat of hydrogenation: the heat given off ΔH° during catalytic hydrogenation.
– Heats of hydrogenation can be measured about as easily as heats of combustion, yet they are smaller numbers and provide more accurate energy differences.
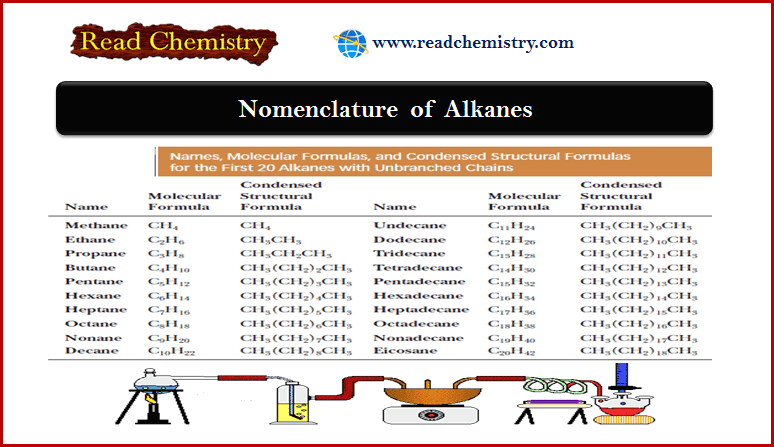
(1) Heats of Hydrogenation of Alkenes
– When an alkene is treated with hydrogen in the presence of a platinum catalyst, hydrogen adds to the double bond, reducing the alkene to an alkane.
– Hydrogenation is mildly exothermic, evolving about 80 to 120 kJ (20 to 30 kcal) of heat per mole of hydrogen consumed.
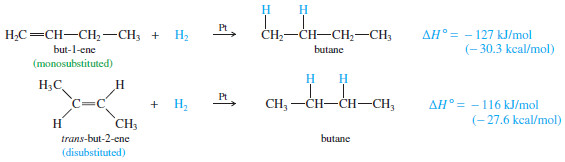
– Consider the hydrogenation of but-1-ene and trans-but-2-ene:
– The following Figure shows these heats of hydrogenation on a reaction-energy diagram.
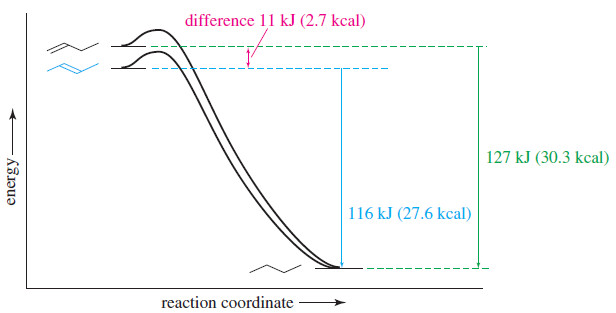
– The difference in the stabilities of but 1-ene and trans-but-2-ene is the difference in their heats of hydrogenation.
– trans-But-2-ene is more stable by
126.8 kJ/mol – 115.6 kJ/mol = 11.2 kJ/mol (2.7 kcal/mol)
(2) Substitution Effects in Alkenes
– An 11 kJ mol (2.7 kcal/mol) stability difference is typical between a monosubstituted alkene (but-1-ene) and a trans-disubstituted alkene (trans-but-2-ene).
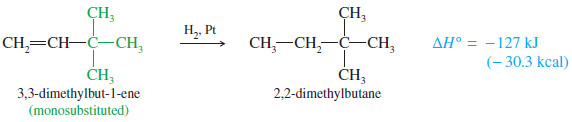
– In the following equations, we compare the monosubstituted double bond of 3-methylbut-1-ene with the trisubstituted double bond of 2 methylbut-2-ene.
– The trisubstituted alkene is more stable by 14 kJ/mol (3.4 kcal/mol).
– To be completely correct, we should compare heats of hydrogenation only for compounds that give the same alkane, as 3-methylbut-1-ene and 2-methylbut-2-ene do.
– However, most alkenes with similar substitution patterns give similar heats of hydrogenation.
– For example, 3,3-dimethylbut-1-ene (below) hydrogenates to give a different alkane than does 3 methylbut-1-ene or but-1-ene (above); yet these three monosubstituted alkenes have similar heats of hydrogenation because the alkanes formed have similar energies.
– In effect, the heat of hydrogenation is a measure of the energy content of the pi bond.
– In practice, we can use heats of hydrogenation to compare the stabilities of different alkenes as long as they hydrogenate to give alkanes of similar energies.
– Most acyclic alkanes and unstrained cycloalkanes have similar energies, and we can use this approximation.
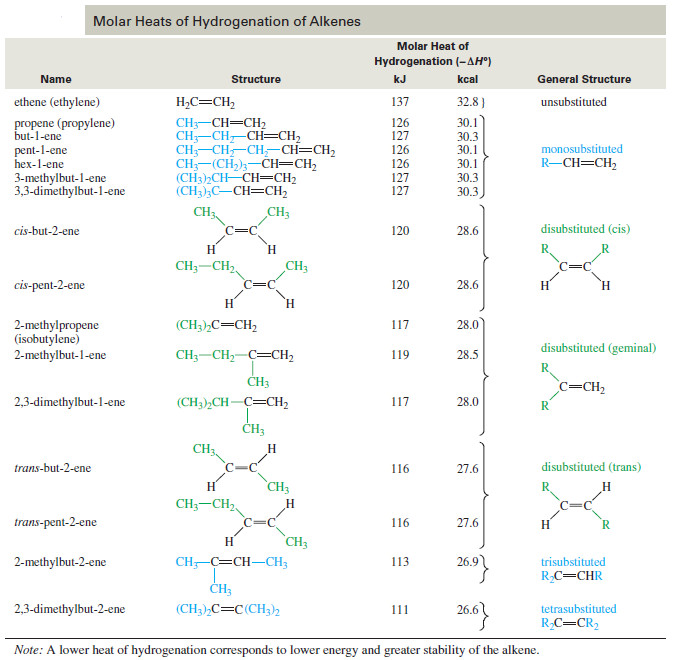
– The following Table shows the heats of hydrogenation of a variety of alkenes with different substitution.
– The compounds are ranked in decreasing order of their heats of hydrogenation, that is, from the least stable double bonds to the most stable.
– Note that the values are similar for alkenes with similar substitution patterns.
– The most stable double bonds are those with the most alkyl groups attached.
– For example, hydrogenation of ethylene (no alkyl groups attached) evolves 137 kJ/ mol, while propene and pent-1-ene (one alkyl group for each) give off 126 kJ/ mol.
– Double bonds with two alkyl groups hydrogenate to produce about 116–120 kJ/mol.
– Three or four alkyl substituents further stabilize the double bond, as with 2-methylbut-2-ene (trisubstituted, 113 kJ/ mol) and 2,3-dimethylbut-2-ene (tetrasubstituted, 111 kJ mol).
– The values in The previous Table confirm Zaitsev’s rule (Saytzeff’s rule):
More substituted double bonds are usually more stable.
– In other words, the alkyl groups attached to the double-bonded carbons stabilize the alkene.
– Two factors are probably responsible for the stabilizing effect of alkyl groups on a double bond.
– Alkyl groups are electron-donating, and they contribute electron density to the pi bond.
– In addition, bulky substituents like alkyl groups are best situated as far apart as possible.
– In an alkane, they are separated by the tetrahedral bond angle, about 109.5°.
– A double bond increases this separation to about 120°.
– In general, alkyl groups are separated best by the most highly substituted double bond.
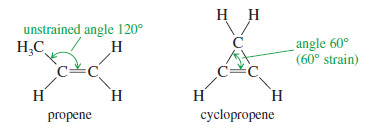
– This steric effect is illustrated in the following Figure for two double-bond isomers (isomers that differ only in the position of the double bond).
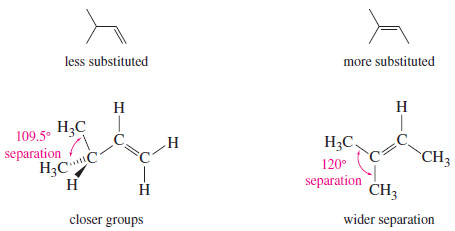
– The isomer with the monosubstituted double bond separates the alkyl groups by only 109.5°, while the trisubstituted double bond separates them by about 120°.
(3) Energy Differences in cis-trans Isomers
– The heats of hydrogenation in the preivous Table show that trans isomers are generally more stable than the corresponding cis isomers.
– This trend seems reasonable because the alkyl substituents are separated farther in trans isomers than they are in cis isomers.
– The greater stability of the trans isomer is evident in the pent-2-enes, which show a 4 kJ/mol (1.0 kcal/mol) difference between the cis and trans isomers.
– A 4 kJ mol difference between cis and trans isomers is typical for disubstituted alkenes.
– The following Figure summarizes the relative stabilities of alkenes, comparing them with ethylene, the least stable of the simple alkenes.
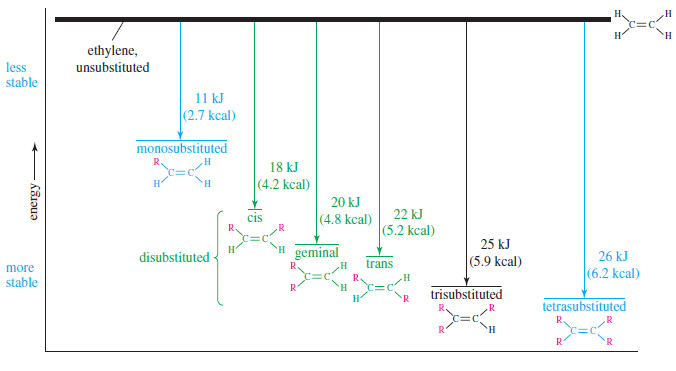
Geminal isomers, CR2 = CH2, tend to fall between the cis and trans isomers in energy, but the differences are not as predictable as the cis/trans differences
(4) Stability of Cycloalkenes
– Most cycloalkenes react like acyclic (noncyclic) alkenes.
– The presence of a ring makes a major difference only if there is ring strain, either because of a small ring or because of a trans double bond.
– Rings that are five-membered or larger can easily accommodate double bonds, and these cycloalkenes react much like straight-chain alkenes.
– Three and four-membered rings show evidence of ring strain, however.
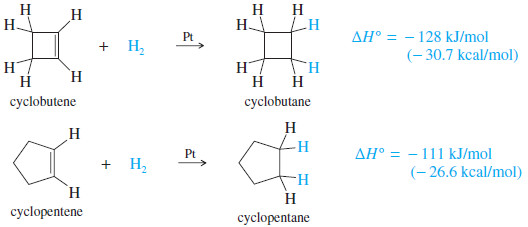
Cyclobutene
Cyclobutene has a heat of hydrogenation of -128 kJ/mol (-30.7 kcal>mol), compared with -111 kJ/mol (-26.6 kcal/mol) for cyclopentene.
– The double bond in cyclobutene has about 17 kJ mol of extra ring strain (in addition to the ring strain in cyclobutane) by virtue of the small ring.
– The 90° bond angles in cyclobutene compress the angles of the sp2 hybrid carbons (normally 120°) more than they compress the sp3 hybrid angles (normally 109.5°) in cyclobutane.
– The extra ring strain in cyclobutene makes its double bond more reactive than a typical double bond.
Cyclopropene
– Cyclopropene has bond angles of about 60°, compressing the bond angles of the carbon–carbon double bond to half their usual value of 120°.
– The double bond in cyclopropene is highly strained
– Many chemists once believed that a cyclopropene could never be made because it would snap open (or polymerize) immediately from the large ring strain.
– Cyclopropene was eventually synthesized, however, and it can be stored in the cold.
– Cyclopropenes were still considered to be strange, highly unusual compounds.
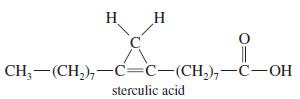
– Natural-product chemists were surprised when they found that the kernel oil of Sterculia foelida, a tropical tree, contains sterculic acid, a carboxylic acid with a cyclopropene ring.
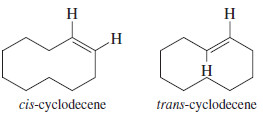
Trans Cycloalkenes
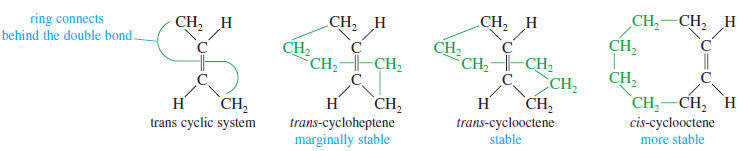
– Another difference between cyclic and acyclic alkenes is the relationship between cis and trans isomers. In acyclic alkenes, the trans isomers are usually more stable; but the trans isomers of small cycloalkenes are rare, and those with fewer than eight carbon atoms are unstable at room temperature.
– The problem with making a trans cycloalkene lies in the geometry of the trans double bond.
– The two alkyl groups on a trans double bond are so far apart that several carbon atoms are needed to complete the ring.
– Try to make a model of trans-cyclohexene, being careful that the large amount of ring strain does not break your models.
– Trans-Cyclohexene is too strained to be isolated, but trans-cycloheptene can be isolated at low temperatures.
– Trans-Cyclooctene is stable at room temperature, although its cis isomer is still more stable.
– Once a cycloalkene contains at least ten or more carbon atoms, it can easily accommodate a trans double bond.
– For cyclodecene and larger cycloalkenes, the trans isomer is nearly as stable as the cis isomer.
(5) Bredt’s Rule
– We have seen that a trans cycloalkene is not stable unless there are at least eight carbon atoms in the ring. An interesting extension of this principle is called Bredt’s rule.
BREDT’S RULE:
A bridged bicyclic compound cannot have a double bond at a bridgehead position unless one of the rings contains at least eight carbon atoms.
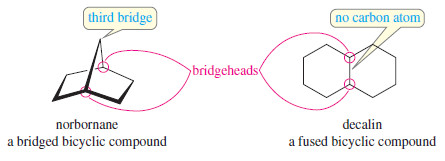
– Let’s review exactly what Bredt’s rule means. A bicyclic compound is one that contains two rings.
– The bridgehead carbon atoms are part of both rings, with three links connecting them.
– A bridged bicyclic compound has at least one carbon atom in each of the three links between the bridgehead carbons.
– In the following examples, the bridgehead carbon atoms are circled in red.
– If there is a double bond at the bridgehead carbon of a bridged bicyclic system, one of the two rings contains a cis double bond and the other must contain a trans double bond.
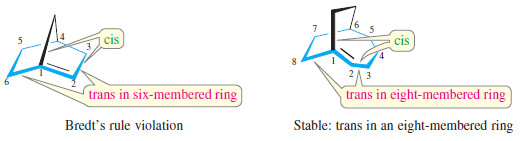
– For example, the following structures show that norbornane contains a fivemembered ring and a six-membered ring.
– If there is a double bond at the bridgehead carbon atom, the five-membered ring contains a cis double bond and the six-membered ring contains a trans double bond. This unstable arrangement is called a “Bredt’s rule violation.”
– If the larger ring contains at least eight carbon atoms, then it can contain a trans double bond and the bridgehead double bond is stable.
– In general, compounds that violate Bredt’s rule are not stable at room temperature.
– In a few cases, such compounds (usually with seven carbon atoms in the largest ring) have been synthesized at low temperatures.
Solved problem
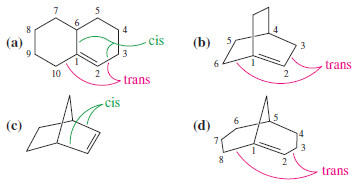
Which of the following alkenes are stable?
SOLUTION:
– Compound (a) is stable. Although the double bond is at a bridgehead, it is not a bridged bicyclic system. The trans double bond is in a 10-membered ring.
– Compound (b) is a Bredt’s rule violation and is not stable. The largest ring contains six carbon atoms, and the trans double bond cannot be stable in this bridgehead position.
– Compound (c) (norbornene) is stable. The (cis) double bond is not at a bridgehead carbon.
– Compound (d) is stable. Although the double bond is at the bridgehead of a bridged bicyclic system, there is an eight-membered ring to accommodate the trans double bond.