Hydrocarbons: Infrared Spectroscopy of Hydrocarbons
Infrared Spectroscopy of Hydrocarbons
– Hydrocarbons contain only carbon–carbon bonds and carbon–hydrogen bonds.
– An infrared spectrum does not provide enough information to identify a structure conclusively (unless an authentic spectrum is available to compare “fingerprints”), but the absorptions of the carbon-carbon and carbon-hydrogen bonds can indicate the presence of double and triple bonds.
Carbon–Carbon Bond Stretching
– Stronger bonds generally absorb at higher frequencies because of their greater stiffness.
- Carbon-carbon single bonds C-C absorb around 1200 cm-1
- C=C double bonds absorb around 1660 cm-1
- C≡C triple bonds absorb around 2200 cm-1
– Carbon-carbon bond stretching frequencies in hydrocarbons:
– As discussed for the octane spectrum, C-C single bond absorptions (and most other absorptions in the fingerprint region) are not very reliable.
– We use the fingerprint region primarily to confirm the identity of an unknown compound by comparison with an authentic spectrum.
– The absorptions of C=C double bonds, however, are useful for structure determination.
– Most unsymmetrically substituted double bonds produce observable stretching absorptions in the region of 1600 to 1680 cm-1.
– The specific frequency of the double bond stretching vibration depends on whether there is another double bond nearby.
– When two double bonds are one bond apart (as in cyclohexa 1,3-diene on the following figure) they are said to be conjugated.
– As we will see in Chapter 15, conjugated double bonds are slightly more stable than isolated double bonds because there is a small amount of pi bonding between them.
– This overlap between the pi bonds leaves a little less electron density in the double bonds themselves.
– As a result, they are a little less stiff and vibrate a little more slowly than an isolated double bond.
– Isolated double bonds absorb around 1640 to 1680 cm-1, while conjugated double bonds absorb around 1620 to 1640 cm-1.
Effect of conjugation
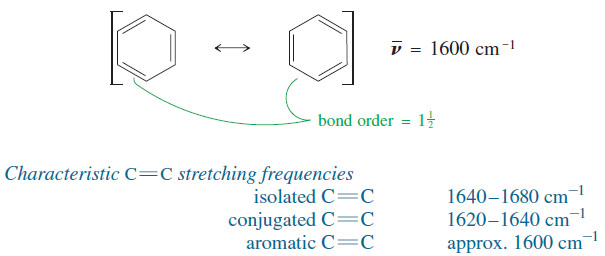
– The effect of conjugation is even more pronounced in aromatic compounds, which have three conjugated double bonds in a six-membered ring.
– Aromatic C=C bonds are more like 3/2 bonds than true double bonds, and their reduced pi bonding results in less stiff bonds with lower stretching frequencies, around 1600 cm-1.
– Carbon-carbon triple bonds in alkynes are stronger and stiffer than carbon-carbon
single or double bonds, and they absorb infrared light at higher frequencies.
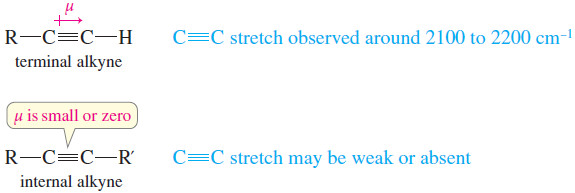
– Most alkyne C≡C triple bonds have stretching frequencies between 2100 and 2200 cm-1.
– Terminal alkynes usually give sharp C≡C stretching signals of moderate intensity.
– The C≡C stretching absorption of an internal alkyne may be weak or absent, however, due to the symmetry of the disubstituted triple bond with a very small or zero dipole moment>
Carbon–Hydrogen Bond Stretching in hydrocarbons
– Alkanes, alkenes, and alkynes also have characteristic C-H stretching frequencies.
– Carbon–hydrogen bonds involving sp3 hybrid carbon atoms generally absorb at frequencies just below (to the right of) 3000 cm-1.
– Those involving sp2 hybrid carbons absorb just above (to the left of) 3000 cm-1.
– We explain this difference by the amount of (s) character in the carbon orbital used to form the bond.
– The (s) orbital is closer to the nucleus than the (p) orbitals, and stronger, stiffer bonds result from orbitals with more (s) character.
– Even if an alkene’s C=C absorption is weak or absent, the unsaturated C-H stretch above 3000 cm-1 reveals the presence of the double bond.
– An sp3 orbital is one-fourth s character, and an sp2 orbital is one-third (s) character.
– We expect the bond using the sp2 orbital to be slightly stronger, with a higher vibration
frequency.
The C-H bond of a terminal alkyne is formed using an sp hybrid orbital, with about one-half (s) character.
This bond is stiffer than a C-H bond using an sp2 or sp2 carbon, and it absorbs at a higher frequency: about 3000 cm-1.
Interpreting the IR Spectra of Hydrocarbons
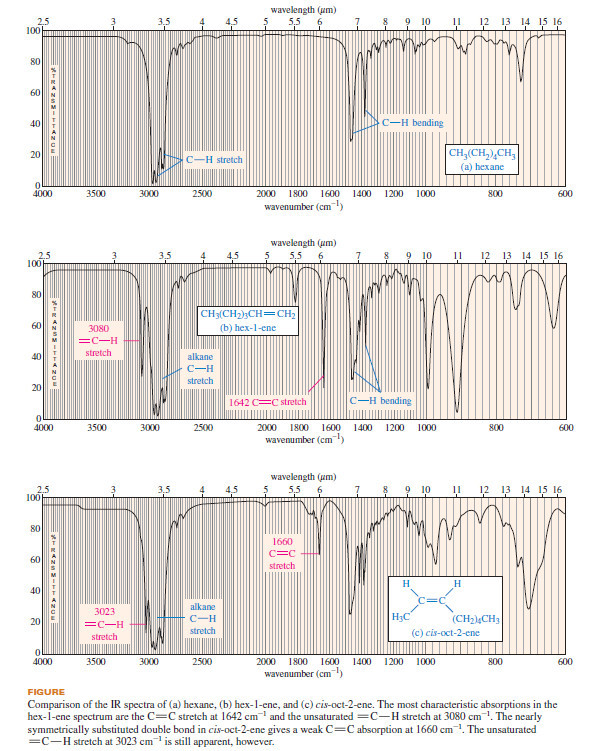
– The following figure compares the IR spectra of hexane, hex-1-ene, and cis-oct-2-ene.
– The hexane spectrum is similar to that of n-octane.
– The C-H stretching frequencies form a band between 2800 and 3000 cm-1 and the bands in the fingerprint region are due to the bending vibrations.
– This spectrum simply indicates the absence of any IR-active functional groups.
– The spectrum of hex-1-ene, shows additional absorptions characteristic of a double bond.
– The C-H stretch at 3080 cm-1 corresponds to the alkene=C-H bonds involving sp2 hybrid carbons. The absorption at 1642 cm-1 results from the stretching of the C=C double bond.
(The small peak at 1820 cm-1 is likely an overtone at double the frequency of the intense peak at 910 cm-1.)
– The spectrum of cis-oct-2-ene (Figure c) resembles the spectrum of hex-1-ene, except that the C=C stretching absorption at 1660 cm-1 is very weak in cis-oct-2-ene because the disubstituted double bond has a very small dipole moment.
Even if the C=C stretching absorption is weak or absent, the unsaturated C-H stretching absorption just above 3000 cm-1 still suggests the presence of an alkene double bond.
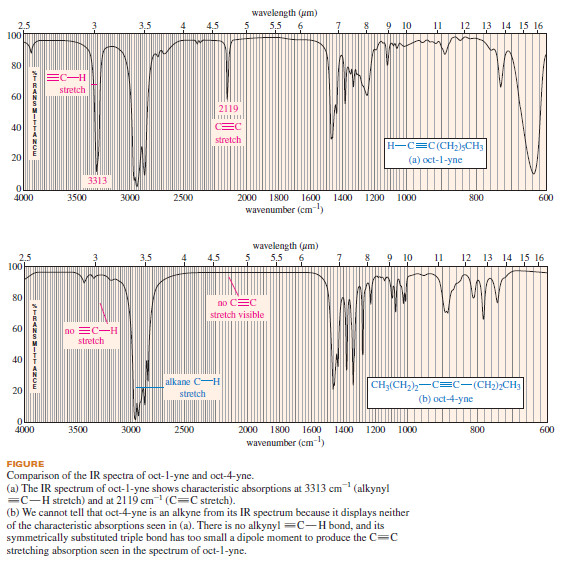
– The following figure compares the IR spectra of oct-1-yne and oct-4-yne.
– In addition to the alkane absorptions, the oct-1-yne spectrum shows sharp peaks at 3313 cm-1 and 2219 cm-1
– The absorption at 3313 cm-1 results from stretching of the stiff ≡C-H bond formed by the sp hybrid alkyne carbon.
– The 2119 cm-1 absorption results from the stretching of the C≡C triple bond.
– The spectrum of oct-4-yne is not very helpful. Since there is no acetylenic hydrogen, there is no ≡C-H stretching absorption around 3300cm-1
– There is no visible C≡C stretching absorption around 2100 to 2200 cm-1 either because the disubstituted triple bond has a very small dipole moment.
– This spectrum fails to alert us to the presence of a triple bond.