Examples of isotopes
– In this subject, we will discuss 7 Examples of isotopes
Definition of isotopes
Isotopes may be defined as :
(1) The atoms of an element that have the same number of protons and different number of neutrons are called Isotopes.
(2) The atoms of an element that have the same atomic number but different atomic masses or mass numbers.
Examples of isotopes
– Since isotopes of an element have the same atomic number, each of these contains an equal number of protons.
– They have different atomic masses which is accounted for by the different number of neutrons present in the nucleus.
– Thus the isotopes of an element are characterized by different number of neutrons in the nucleus.
– The atomic structure of an isotope with atomic number Z and mass number A (atomic mass in amu) can be given as follows:
(1) The number of extranuclear electrons = Z
(2) The number of protons in the nucleus = Z
(3) The mass number A is equal to the total number of protons (Z) and neutrons (N) in the nucleus. That is,
A = Z + N
∴ N = A – Z
Isotopes of Hydrogen
– Famous examples of isotopes are isotopes of hydrogen
– There are three isotopes of hydrogen: protium 1H1, deuterium 2H1 or D, and tritium 3H1 or T.
– Protium is by far the most abundant in natural hydrogen, deuterium about 0.015%, and tritium only one out of 10,000,000 hydrogen atoms.
– The atomic number of the three isotopes of hydrogen is 1, while their mass numbers are: protium 1, deuterium 2, and tritium 3.
– Therefore each of the three isotopes has one extranuclear electron and one proton in the nucleus.
– The nucleus of protium is made of one proton only, while the number of neutrons (A – Z) present in deuterium is 2 – 1 = 1, and in tritium 3– 1 = 2.
– The structure of the three isotopes of hydrogen can be pictorially represented as:
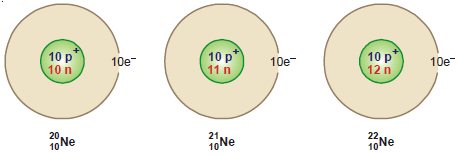
Isotopes of Neon
– Neon has been found to consist of three isotopes: 20Ne10, 21Ne10, and 22Ne10. Their percentage abundance is:
20Ne 21Ne 22Ne
90.92% 0.257% 8.82%
– The atomic number of the three isotopes of neon is 10, while their mass numbers are 20, 21, and 22 respectively.
– Therefore each of these isotopes has ten extranuclear electrons and ten protons in the nucleus.
– The number of neutrons (A – Z) are: 20Ne, 20 – 10 = 10; 21Ne, 21 – 10 = 11; 22Ne, 22 – 10 = 12.
– The atomic structure of the isotopes of neon can, therefore, be represented pictorially as:
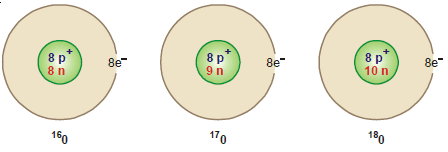
Isotopes of Oxygen
– Oxygen has three isotopes: 16O8, 17O8, and 18O8 These are found with relative abundances of 99.759, 0.037, and 0.204 respectively.
– The atomic number of the above three isotopes of oxygen is 8 while their mass numbers are 16, 17, and 18.
– Therefore each isotope has 8 extranuclear electrons and 8 protons in the nucleus.
– The number of neutrons (A – Z) in the three isotopes is:
16O 16 – 8 = 8 neutrons
17O 17 – 8 = 9 neutrons
18O 18 – 8 = 10 neutrons
– The complete atomic structure of the isotopes of oxygen can be pictorially represented as:
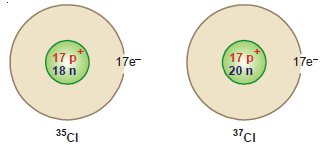
Isotopes of Chlorine
– Chlorine is a mixture of two isotopes: 35Cl17, and 35Cl17 Their percentage abundance is 75.53 and 24.47 respectively.
– The atomic number of the two isotopes of chlorine is 17 while their mass numbers are 35 and 37.
– Therefore each isotope has 17 extranuclear electrons and 17 protons in the nucleus.
– The number of neutrons (A – Z) in these isotopes is:
35Cl 35 – 17 = 18 neutrons
37Cl 37 – 17 = 20 neutrons
– The atomic structure of the isotopes of chlorine can be pictorially represented as:
Isotopes of Uranium
– There are three isotopes of uranium: 234U92, 235U92, 238U92,
– Natural uranium consists almost entirely of 238U, with about 0.72% of 235U and 0.006% of 234U.
– These isotopes are particularly important in atomic energy.
– The atomic number of the three isotopes of uranium is 92 and their mass numbers are 238, 235, and 234.
– Thus each isotope has 92 extranuclear electrons and 92 protons.
– The number of neutrons (A – Z) in these isotopes is:
238U 238 – 92 = 146 neutrons
235U 235 – 92 = 143 neutrons
234U 234 – 92 = 142 neutrons
– The atomic structure of the three isotopes of uranium may be represented as:
Isotopes of Carbon
– Carbon has three naturally occurring isotopes.
– The isotopes of carbon are :
– Carbon-12: which constitutes 98.89 of all carbon atoms and serves as the standard for the atomic mass scale
– Carbon-13: which is the only magnetic isotope, making it very important for structural studies of compounds containing carbon
– Carbon-14: which is produced by cosmic rays bombarding the atmosphere.
– Carbon-14 is radioactive, with a half-life of 5760 years.
– The amount of carbon-14 remaining in historical artifacts can be used to estimate their age.
Notes on Examples of isotopes
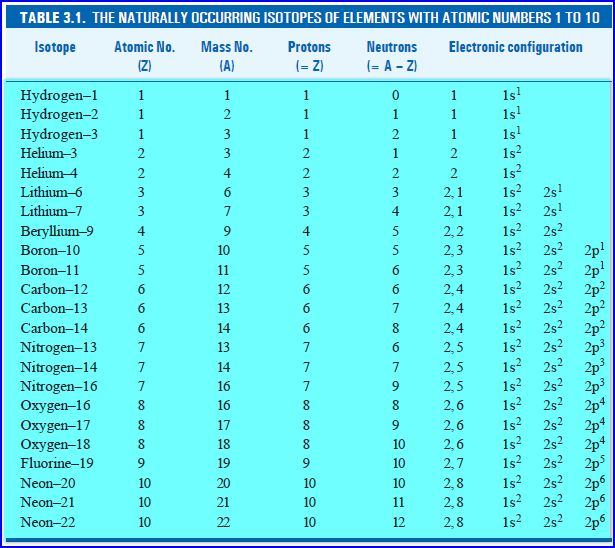
– Almost every element in nature exists as a mixture of isotopes.
– The isotopes of the elements with atomic numbers 1 to 10 and their structure are listed in the Table below.
– It may be noted that some elements e.g., fluorine, are monoisotopic.
– These are found in nature only as a single isotope.
– About 20 elements are monoisotopic.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.





https://www.youtube.com/redirect?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ae/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.at/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.be/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.bg/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.by/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ca/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ch/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.cl/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.bw/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.cr/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.th/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.ug/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.uk/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.ve/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.za/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.au/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.br/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.co/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.cu/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.do/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.ec/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.eg/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.gh/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.gt/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.mx/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.my/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.pe/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.ph/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.pk/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.pr/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.py/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.sa/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.sg/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.tr/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.tw/url?q=https%3A%2Flearnchemistry12.com
https://cse.google.com.ua/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.uy/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.vn/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.cz/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.de/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.dk/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ee/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.de/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.de/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.jp/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.co.jp/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.es/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.es/url?q=https%3A%2Flearnchemistry12.com
https://images.google.com.br/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.br/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.uk/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.uk/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.fr/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.fr/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.it/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.it/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ru/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.ru/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.pl/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.pl/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ca/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.ca/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.nl/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.nl/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.in/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.in/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.tw/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.tw/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.id/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.co.id/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.cz/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.ua/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.ua/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.mx/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.mx/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.au/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.au/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.be/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.be/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.th/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.co.th/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.tr/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.tr/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.pt/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.pt/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.gr/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.gr/url?q=https%3A%2F%2Flearnchemistry12.com
https//maps.google.ro/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.ro/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.ch/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ch/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.at/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.at/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.dk/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.dk/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.hu/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.hu/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.fi/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.fi/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.vn/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.cl/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.cl/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.bg/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.bg/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.my/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.my/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.il/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.co.il/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.ie/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.ie/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.pe/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.co/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.co/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.sg/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.sg/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.sg/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.sk/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.sk/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.co.nz/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.co.za/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.za/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.hr/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.hr/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.co.ve/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.ve/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ae/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.ae/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.sa/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.sa/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.si/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.si/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.hk/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.hk/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.eg/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.eg/url?q=https%3A%2F%2Fwww.moviesneek.com
https://maps.google.lt/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.lv/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.lv/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.pk/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.ec/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.ec/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.pr/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.lu/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.lu/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.do/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.com.do/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.cr/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.bw/url?q=httpss%3A%2F%2Flearnchemistry12.com
https://www.google.co.bw/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.is/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.cu/url?q=httpss%3A%2F%2Flearnchemistry12.com
https://maps.google.com.cu/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.ee/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ee/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.gt/url?q=https%3A%2Flearnchemistry12.com
https://maps.google.com.gt/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.ug/url?q=httpss%3A%2Flearnchemistry12.com
https://images.google.co.ug/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.co.ma/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.py/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.py/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.py/url?q=httpss%3A%2F%2Flearnchemistry12.com
https://maps.google.com.gh/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.bj/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ml/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ml/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.ls/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.ls/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ms/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.ms/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ms/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.dm/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.dm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.sb/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.cv/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.vi/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.vi/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.tl/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.mm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.mm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ga/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.so/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.kg/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.kg/url?q=https%3A%2F%2Flearnchemistry12.com
https://www.google.kg/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.nr/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.nr/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.mv/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.mv/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.sc/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.sc/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ad/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.ad/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.bz/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.bz/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.to/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ws/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.ws/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.com.bn/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.bn/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.bn/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.sh/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.sh/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.sh/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.uz/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.zm/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.co.zm/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.co.zm/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.com.ag/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.ag/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.co.ck/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.pg/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.je/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.je/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.gl/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.gp/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.com.nf/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.sr/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.sr/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.nu/url?q=https%3A%2F%2Flearnchemistry12.com
https://maps.google.bt/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.bt/url?q=https%3A%2F%2Flearnchemistry12.com
https://images.google.bt/url?q=https%3A%2F%2Flearnchemistry12.com
https://cse.google.ac/url?q=https%3A%2F%2Flearnchemistry12.com