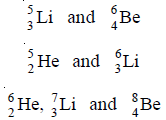Difference between Isotopes, Isobar, and Isotones
In this subject, the Difference between Isotopes, Isobar, and Isotones will be discussed with some Common Examples for all types.
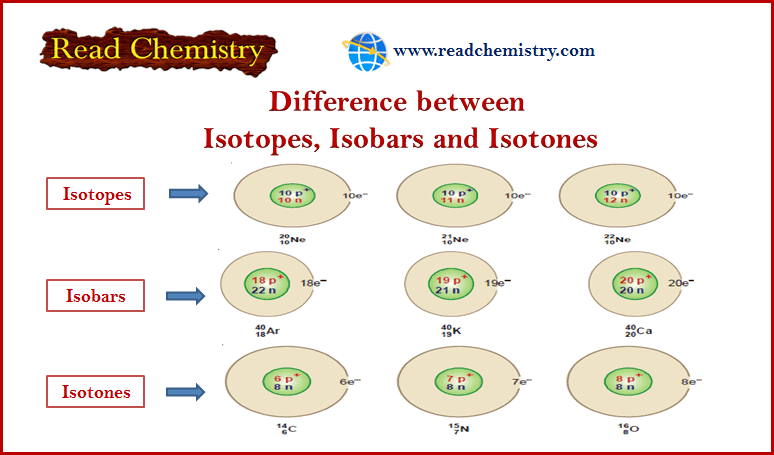
What are Isotopes?
Isotopes may be defined as:
(1) The atoms of an element that have the same number of protons and different number of neutrons are called Isotopes.
(2) The atoms of an element that have the same atomic number but different atomic masses or mass numbers.
– Since isotopes of an element have the same atomic number, each of these contains an equal number of protons.
– They have different atomic masses which is accounted for by the different number of neutrons present in the nucleus.
– Thus the isotopes of an element are characterized by different number of neutrons in the nucleus.
Examples of Isotopes
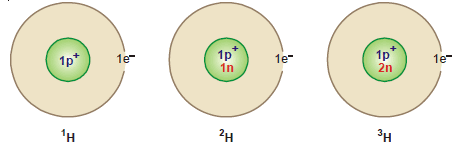
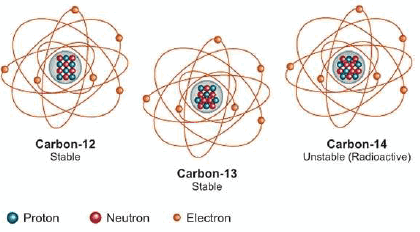
(1) Hydrogen: There are three isotopes of hydrogen: protium 1H1, deuterium 2H1 or D , and tritium 3H1 or T.
(2) Neon: Neon has been found to consist of three isotopes: 20Ne10, 21Ne10, 22Ne10
(3) Oxygen: 16O8, 17O8, 18O8
(4) Chlorine: 35Cl17,35Cl17
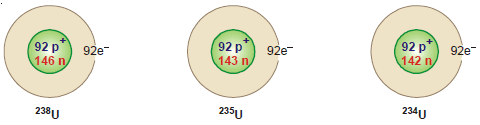
(5) Uranium: There are three isotopes of uranium: 234U92, 235U92, 238U92,
(6) Carbon: There are three isotopes of uranium: 12C6,13C6, 14C6
What are Isobars?
– Isobars are atoms that have the same mass number but different atomic numbers are called isobars.
– The word isobar meaning ‘equally heavy’ is taken from the Greek isos = equal, and barys = heavy.
Examples of isobars
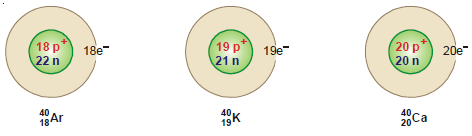
– For example, 40 Ar18, 40K19, and 40Ca20 are isobaric atoms.
– Similarly, 235U92, 235Np93, 235Pu94 are isobars.
– Since isobars have the same mass number, the number of protons plus neutrons in the nucleus in each of these is equal.
– The number of protons is given by atomic number (Z), the number of neutrons is, therefore, (A – Z) where A is the mass number.
– The number of extranuclear electrons is equal to (Z).
– Thus the atomic structure of the isobars 40Ar18 , 40K19 and 40Ca20 is shown below:
What are Isotones?
– Isotones are Atoms that have different atomic number and different atomic masses but the same number of neutrons.
– Isotones are different elements having entirely different atomic structures.
– They have different physical and chemical properties.
Examples of isotones
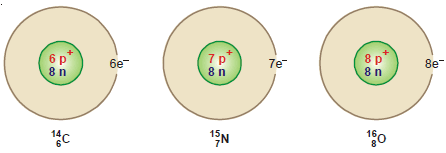
(1) 14C6 , 15N7 , 16O8 are isotones since each contains eight neutrons
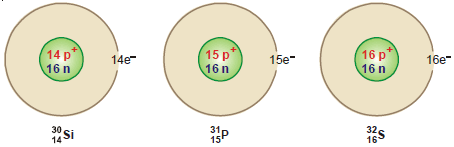
(2) 30Si14 , 31P15 , 32S16 are isotones. Each contains sixteen neutrons.
(3) Some other examples of isotones are:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.