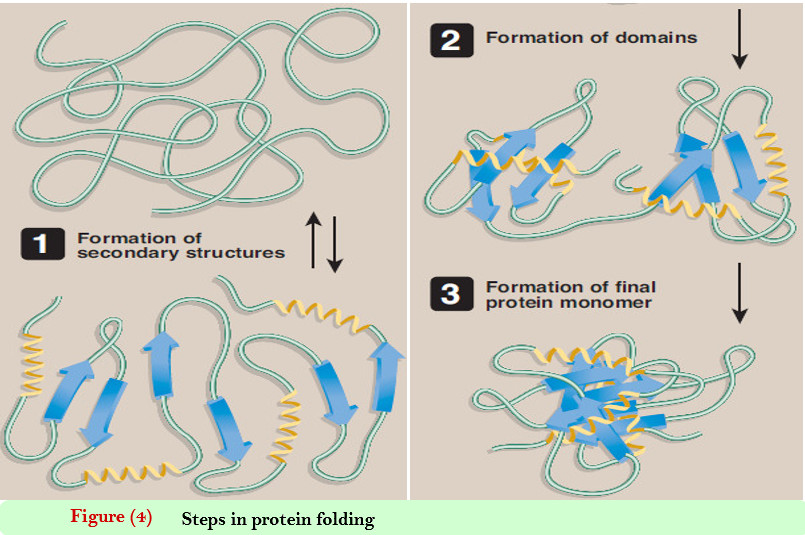
Protein Secondary Structure
– In the last topic in Biochemistry section , we discussed The Protein Primary Structure but now we will talk about Protein Secondary Structure.
Secondary structure of protein
– The polypeptide backbone does not assume a random three-dimensional structure, but instead generally forms regular arrangements of amino acids that are located near to each other in the linear sequence.
– These arrangements are termed the secondary structure of the polypeptide.
– The α-helix, β-sheet, and β-bend (β-turn) are examples of secondary structures frequently encountered in proteins.
– [Note: The collagen α-chain helix, another example of Protein secondary structure]
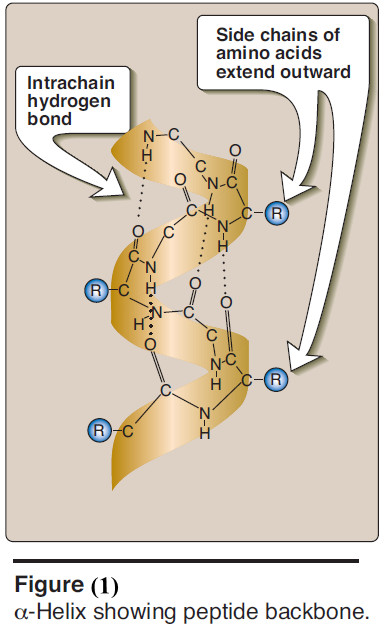
A. α-Helix
– Several different polypeptide helices are found in nature, but the α-helix is the most common.
– It is a spiral structure, consisting of a tightly packed, coiled polypeptide backbone core, with the side chains of the component amino acids extending outward from the central axis to avoid interfering sterically with each other (Figure 1).
– A very diverse group of proteins contains α-helices.
– For example, the keratins are a family of closely related, fibrous proteins whose structure is nearly entirely α-helical.
– They are a major component of tissues such as hair and skin, and their rigidity is determined by the number of disulfide bonds between the constituent polypeptide chains.
– In contrast to keratin, myoglobin, whose structure is also highly α-helical, is a globular, flexible molecule.
(1) Hydrogen bonds
– An α-helix is stabilized by extensive hydrogen bonding between the peptide-bond carbonyl oxygens and amide hydrogens that are part of the polypeptide backbone (see Figure 1).
– The hydrogen bonds extend up and are parallel to the spiral from the carbonyl oxygen of one peptide bond to the – NH – group of a peptide linkage four residues ahead in the polypeptide.
– This ensures that all but the first and last peptide bond components are linked to each other through intrachain hydrogen bonds.
– Hydrogen bonds are individually weak, but they collectively serve to stabilize the helix.
(2) Amino acids per turn
– Each turn of an α-helix contains 3.6 amino acids.
– Thus, amino acid residues spaced three or four residues apart in the primary sequence are spatially close together when folded in the α-helix.
(3) Amino acids that disrupt an α-helix
– Proline disrupts an α-helix because its secondary amino group is not geometrically compatible with the right handed spiral of the α-helix.
– Instead, it inserts a kink in the chain, which interferes with the smooth, helical structure.
– Large numbers of charged amino acids (for example, gluta- mate, aspartate, histidine, lysine, or arginine) also disrupt the helix by forming ionic bonds, or by electrostatically repelling each other.
– Finally, amino acids with bulky side chains, such as tryptophan, or amino acids, such as valine or isoleucine, that branch at the β-carbon (the first carbon in the R-group, next to the α-carbon) can interfere with formation of the α-helix if they are present in large numbers.
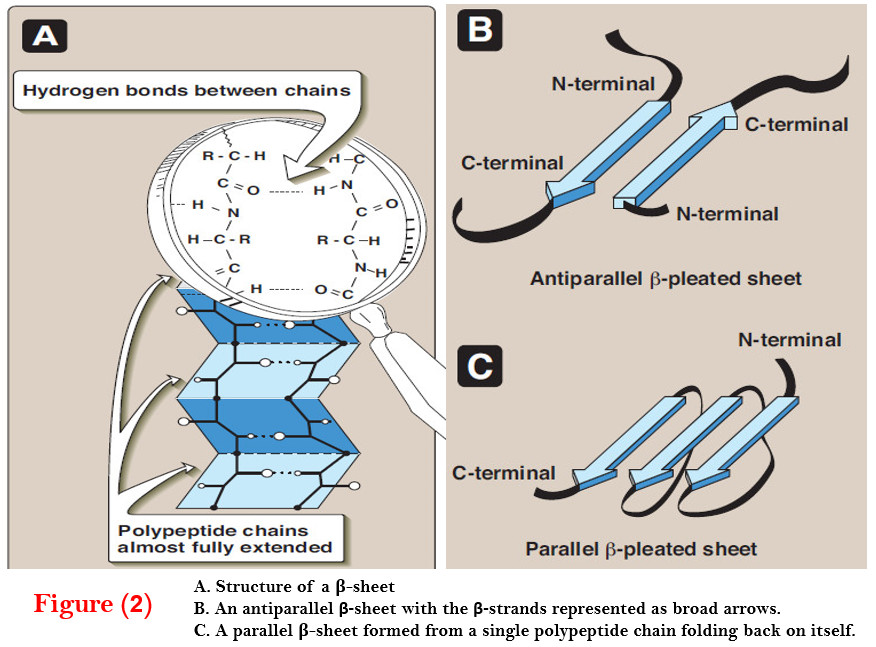
B. β-Sheet
– The β-sheet is another form of secondary structure in which all of the peptide bond components are involved in hydrogen bonding (Figure 2).
– The surfaces of β-sheets appear “pleated,” and these structures are, therefore, often called “β pleated sheets.”
– When illustrations are made of protein structure, β-strands are often visualized as broad arrows (Figure 2B).
(1) Comparison of a β-sheet and an α-helix
– Unlike the α-helix, β-sheets are composed of two or more peptide chains (β-strands), or segments of polypeptide chains, which are almost fully extended.
– Note also that in β-sheets the hydrogen bonds are perpendicular to the polypeptide backbone (see Figure 2A).
(2) Parallel and antiparallel sheets
– A β-sheet can be formed from two or more separate polypeptide chains or segments of polypeptide chains that are arranged either antiparallel to each other (with the N-terminal and C-terminal ends of the β-strands alternating as shown in Figure 2B), or parallel to each other (with all the N-termini of the β-strands together as shown in Figure 2C).
– When the hydrogen bonds are formed between the polypeptide backbones of separate polypeptide chains, they are termed interchain bonds.
– A β-sheet can also be formed by a single polypeptide chain folding back on itself (see Figure 2C). In this case, the hydrogen bonds are intrachain bonds.
– In globular proteins, β-sheets always have a right-handed curl, or twist, when viewed along the polypeptide backbone.
– [Note: Twisted β- sheets often form the core of globular proteins.]
C. β-Bends (reverse turns, β-turns)
– β-Bends reverse the direction of a polypeptide chain, helping it form a compact, globular shape.
– They are usually found on the surface of protein molecules, and often include charged residues.
– [Note: β-Bends were given this name because they often connect successive strands of antiparallel β-sheets.]
– β-Bends are generally composed of four amino acids, one of which may be proline—the amino acid that causes a “kink” in the polypeptide chain.
– Glycine, the amino acid with the smallest R-group, is also frequently found in β-bends.
– β-Bends are stabilized by the formation of hydrogen and ionic bonds.
D. Nonrepetitive secondary structure
– Approximately one half of an average globular protein is organized into repetitive structures, such as the α-helix and/or β-sheet.
– The remainder of the polypeptide chain is described as having a loop or coil conformation.
– These nonrepetitive secondary structures are not “random,” but rather simply have a less regular structure than those described above.
[Note: The term “random coil” refers to the disordered structure obtained when proteins are denatured.]
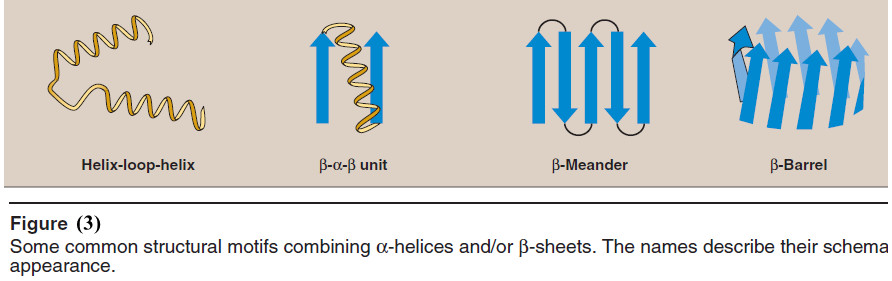
E. Supersecondary structures (motifs)
Globular proteins are constructed by combining secondary structural elements (α-helices, β sheets, nonrepetitive sequences).
– These form primarily the core region—that is, the interior of the molecule.
– They are connected by loop regions (for example, β-bends) at the surface of the protein.
– Supersecondary structures are usually produced by packing side chains from adjacent secondary structural elements close to each other.
– Thus, for example, α-helices and β-sheets that are adjacent in the amino acid sequence are also usually (but not always) adjacent in the final, folded protein.
– Some of the more common motifs are illustrated in Figure (3).
References:
- Lehninger Principles of Biochemistry / David L. Nelson, Michael M. Cox/ 7th ed, 2017.
- Lippincott’s Illustrated Reviews: Biochemistry / Richard A. Harvey, Denise R. Ferrier/ 5th ed, 2011 / Lippincott Williams & Wilkins, USA.
- Harper’s Illustrated Biochemistry /Robert K. Murray, David A. Bender , Kathleen M. Botham / 28th, 2009/ McGraw-Hill Companies, Inc. USA.