Organic Chemistry
How to write and interpret Structural Formulas?
** Organic chemists use a variety of formats to write structural formulas.
** Structural Formulas are written by:
(1) Ball-and-stick model
(2) Electron-dot formula (lewis structure)
(3) Dash formula
(4) Condensed formula
(5) Bond-line formula
** Examples of these types of structural formulas are shown in the following Figure using propyl alcohol as an example:
** An older term for isomers of this type was structural isomers.
** The International Union of Pure and Applied Chemistry (IUPAC) now recommends that use of the term “structural” when applied to constitutional isomers be abandoned.
** Although electron-dot formulas account explicitly for all of the valence electrons in a molecule, they are tedious and time-consuming to write.
** Dash, condensed, and bond-line formulas are therefore used more often.
** Generally it is best to draw unshared electron pairs in chemical formulas, though sometimes they are omitted if we are not considering the chemical properties or reactivity of a compound. When we write chemical reactions, however, we shall see that it is necessary to include the unshared electron pairs when they participate in a reaction. It is a good idea, therefore, to be in the habit of writing unshared electrons pairs.
** You can read full subject about Lewis structure on our website
** In the subject we will talk about the most used structural formulas (Dash formula – Condensed formula – Bond-line formula)
(1) Dash Structural Formulas
** Dash structural formulas have lines that show bonding electron pairs, and include elemental symbols for all of the atoms in a molecule.
** If we look at the ball-and-stick model for propyl alcohol given in the previous Figure and compare it with the electron-dot, dash, and condensed formulas in the same Figure we find that the chain of atoms is straight in those formulas.
** In ball-and-stick model, which corresponds more accurately to the actual shape of the molecule, the chain of atoms is not at all straight.
** Also of importance is this: Atoms joined by single bonds can rotate relatively freely with respect to one another. This relatively free rotation means that the chain of atoms in propyl alcohol can assume a variety of arrangements like these:
** It also means that all of the structural formulas above are equivalent and all represent propyl alcohol.
** Dash structural formulas such as these indicate the way in which the atoms are attached to each other and are not representations of the actual shapes of the molecule. (Propyl alcohol does not have 90o bond angles. It has tetrahedral bond angles.)
** Dash structural formulas show what is called the connectivity of the atoms.
** Constitutional isomers have different connectivities and, therefore, must have different structural formulas.
** Constitutional isomer is different compounds that have the same molecular formula but differ in the sequence in which their atoms are bonded—that is, their connectivity
** Consider the compound called isopropyl alcohol, whose formula we might write in a variety of ways:
** Isopropyl alcohol is a constitutional isomer of propyl alcohol because its atoms are connected in a different order and both compounds have the same molecular formula, C3H8O. In isopropyl alcohol the OH group is attached to the central carbon; in propyl alcohol it is attached to an end carbon.
** In problems you will often be asked to write structural formulas for all the isomers that have a given molecular formula. Do not make the error of writing several equivalent formulas, like those that we have just shown, mistaking them for different constitutional isomers.
(2) Condensed Structural Formulas
** Condensed structural formulas are somewhat faster to write than dash formulas and, when we become familiar with them, they will impart all the information that is contained in the dash structure.
** In condensed formulas all of the hydrogen atoms that are attached to a particular carbon are usually written immediately after the carbon.
** In fully condensed formulas, all of the atoms that are attached to the carbon are usually written immediately after that carbon, listing hydrogens first.
** For example:
** The condensed formula for isopropyl alcohol can be written in four different ways:
Solved Problem(1):Write a condensed structural formula for the compound that follows:
Answer:
(3) Bond-Line Formulas
** The most common type of structural formula used by organic chemists, and the fastest to draw, is the bond-line formula. (Some chemists call these skeletal formulas.)
** The formula in the next figure is a bond-line formula for propyl alcohol.
** The sooner you master the use of bond-line formulas, the more quickly you will be able to draw molecules when you take notes and work problems. And, lacking all of the symbols that are explicitly shown in dash and condensed structural formulas, bond-line formulas allow you to more quickly interpret molecular connectivity and compare one molecular formula with another.
How To Draw Bond-Line Formulas
We apply the following rules when we draw bond-line formulas:
(1) Each line represents a bond.
(2) Each bend in a line or terminus of a line represents a carbon atom, unless another group is shown explicitly.
(3) No Carbons are written for carbon atoms, except optionally for CH3 groups at the end of a chain or branch.
(4) No Hydrogens are shown for hydrogen atoms, unless they are needed to give a three dimensional perspective, in which case we use dashed or solid wedges (as explained in the next section).
(5)The number of hydrogen atoms bonded to each carbon is inferred by assuming that as
many hydrogen atoms are present as needed to fill the valence shell of the carbon, unless
a charge is indicated.
(6) When an atom other than carbon or hydrogen is present, the symbol for that element is
written at the appropriate location (i.e., in place of a bend or at the terminus of the line
leading to the atom).
(7) Hydrogen atoms bonded to atoms other than carbon (e.g., oxygen or nitrogen) are written
explicitly.
** Consider the following examples of molecules depicted by bond-line formulas.
** Bond-line formulas are easy to draw for molecules with multiple bonds and for cyclic molecules, as well. The following are some examples:
Solved Problem(2):Write the bond-line formula for
Strategy and Answer:
First, for the sake of practice, we outline the carbon skeleton, including the OH group as follow:
Then we write the bond-line formula as:
As you gain experience you will likely skip the intermediate steps shown above and proceed directly to writing bond-line formulas.
Three-Dimensional Formulas
** None of the formulas that we have described so far convey any information about how the atoms of a molecule are arranged in space.
** Molecules exist in three dimensions. We can depict three-dimensional geometry in molecules using bonds represented by dashed wedges, solid wedges, and lines.
(1) A dashed wedge: represents a bond that projects behind the plane of the paper.
(2) A solid wedge: represents a bond that projects out of the plane of the paper.
(3) An ordinary line: represents a bond that lies in the plane of the paper.
** For example, the four C-H bonds of methane (CH4) are oriented toward the corners of a regular tetrahedron, with the carbon in the center and an approximately 1098 angle between each C-H bond, as was originally postulated by J. H. van’t Hoff and L. A. Le Bel in 1874.
** The following Figure shows the tetrahedral structure of methane.
** let us consider some guidelines for representing these bonding patterns in three dimensions using dashed and solid wedge bonds. In general for carbon atoms that have only single bonds:
(1) A carbon atom with four single bonds has tetrahedral geometry and can be drawn with two bonds in the plane of the paper separated by approximately 1098, one bond behind the plane using a dashed wedge, and one bond in front of the plane using a solid wedge.
(2) The dashed wedge and solid wedge bonds in tetrahedral geometry nearly eclipse each other when drawn in proper three-dimensional perspective. For carbon atoms with a double or a triple bond:
(3) A carbon atom with a double bond has trigonal planar geometry (Section 1.13) and can be depicted with bonds that are all in the plane of the paper and separated by 1208.
(4) A carbon atom with a triple bond has linear geometry (Section 1.14) and can be depicted with its bonds in the plane of the paper and separated by a 1808 angle. Last, when drawing three-dimensional formulas for molecules:
(5) Draw as many carbon atoms in the plane of the paper as possible using ordinary lines, then use dashed or solid wedge bonds for substituent groups or hydrogen atoms that are needed to show three dimensions.
Some examples of three-dimensional formulas are shown below:
Solved Problem(3):Write a bond-line formula for the following compound showing three dimensions at the carbon bearing the chlorine atom.
Strategy and Answer:
First draw the carbon skeleton, placing as many carbon atoms in the plane of the paper as possible (which is all of them, in this case).
Then add the chlorine atom at the appropriate carbon using a three-dimensional representation.
Reference: Organic chemistry / T.W. Graham Solomons , Craig B.Fryhle , Scott A.snyder , / ( eleventh edition) / 2014.











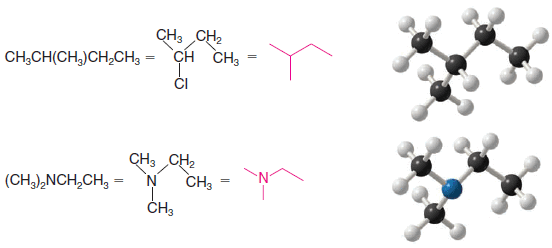









I noticed that under “How To Draw Bond-Line Formulas”, the second compound depicted in the diagram (CH3CH(CH3)CH2CH3) is drawn with a chlorine atom, but I believe this is incorrect.
Otherwise, this was a super helpful resource, thanks!