– In this subject, we will discuss the Hybridization: Definition, Types, Rules, and Examples
– While the formation of simple molecules could be explained adequately by the overlap of atomic orbitals, the formation of molecules of Be, B, and C present problems of greater magnitude having no solution with the previous theory.
– To explain fully the tendency of these atoms to form bonds and the shape or geometry of their molecules, a new concept called Hybridization is introduced.
Atomic orbitals and Hybrid orbitals
– According to this concept, we may mix any number of atomic orbitals of an atom, which differ in energy only slightly, to form new orbitals called Hybrid orbitals.
– The mixing orbitals generally belong to the same energy level (say 2s and 2p orbitals may hybridize).
– The total number of hybrid orbitals formed after mixing is invariably equal to the number of atomic orbitals mixed or hybridized.
- An important characteristic of hybrid orbitals is that:
(1) they are all identical in respect of energy and directional character.
(2) They, however, differ from the original atomic orbitals in these respects.
(3) They may also differ from one another in respect of their arrangement in space. i.e., orientation.
(4) Like pure atomic orbitals, the hybrid orbitals of an atom shall have a maximum of two electrons with opposite spin.
(5) Hybrid orbitals of an atom may overlap with other bonding orbitals (pure atomic or hybrid) on other atoms or form molecular orbitals and hence new bonds.
Definition of Hybridization
- Hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new orbitals equal in number to the mixing orbitals and having the same energy contents and identical shapes.
Rules of Hybridization
– For hybridization to occur, it is necessary for the atom to satisfy the following conditions :
(1) Orbitals on a single atom only would undergo hybridization.
(2) There should be very little difference in energy level between the orbitals mixing to form hybrid orbitals.
(3) The number of hybrid orbitals generated is equal to the number of hybridizing orbitals.
(4) The hybrid orbitals assume the direction of the dominating orbitals.
– For example, if s and p orbitals are to hybridize, the s orbital having no directional character, does not contribute towards the direction when p orbitals determine the directional character of the hybrid orbitals.
(5) It is the orbitals that undergo hybridization and not the electrons.
– For example, four orbitals of an oxygen atom (2 2, 2 2, 2 1, 2 1 ) s px py pz belonging to the second level (i.e., 2s, 2px, 2py, 2pz) can hybridize to give four hybrid orbitals, two of which have two electrons each (as before) and the other two have one electron each.
(6) The electron waves in hybrid orbitals repel each other and thus tend to be farthest apart.
Types of Hybridization
– Since hybridization lends an entirely new shape and orientation to the valence orbitals of an atom, it holds significant importance in determining the shape and geometry of the molecules formed from such orbitals.
– Depending upon the number and nature of the orbitals undergoing by hybridisation, we have various types of hybrid orbitals.
– For instance s, p, and d orbitals of simple atoms may hybridize in the following manner:
- sp Hybridization
- sp2 Hybridization
- sp3 Hybridization
- Hybridization involving (d) orbitals
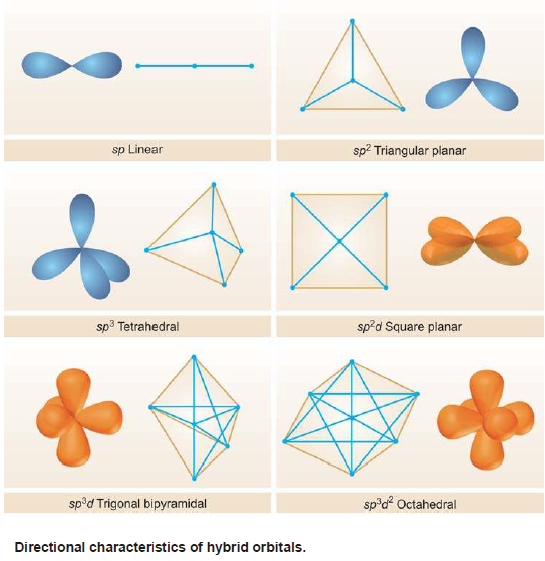
sp Hybridization
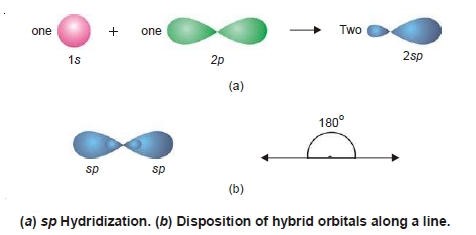
– The mixing of an (s) and a (p) orbital only leads to two hybrid orbitals known as sp hybrid orbitals after the name of an s and a p orbital involved in the process of hybridisation. The process is called sp hybridization.
– In sp hybridization process, Each sp orbital has 50%, s-character, and 50% p-character.
– Orbitals thus generated are the seat of electrons which have a tendency to repel and be farther apart.
– To do so the new orbitals arrange themselves along a line and are, therefore, often referred to as Linear hybrid orbitals.
– This gives an angle of 180º between the axes of the two orbitals.
– The following Figure that an sp orbital has two lobes (a character of p orbital) one of which is farther than the corresponding s or p orbitals and also protrudes farther along the axis.
– It is this bigger lobe that involves itself in the process of an overlap with orbitals of other atoms to form bonds.
– It will be seen later on that the smaller lobes of hybrid orbitals are neglected while considering bond formation.
– Examples of sp Hybridization: BeF2, BeCl2, etc.
sp2 Hybridization
– When an s and two p orbitals mix up to hybridize, there result three new orbitals called sp2 hybrid orbitals (spoken as ‘sp two’).
– In the sp2 hybridization process, Each sp2 hybrid orbital has 33% s-character and 67% p-character.
– As the three orbitals undergoing hybridisation lie in a plane, so do the new orbitals.
– They have to lie farthest apart in a plane which can happen if they are directed at an angle of 120º to one another as shown in Fig. (b).
– It is for this reason that sp2 hybrid orbitals are also called Trigonal hybrids, the process being referred to as Trigonal hybridization.
– The sp2 hybrid orbitals resemble in shape with that of sp hybrid orbitals but are slightly fatter.
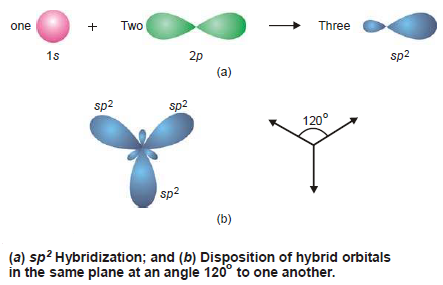
– Examples of sp2 Hybridization: BF3, NO3–, etc.
sp3 Hybridization
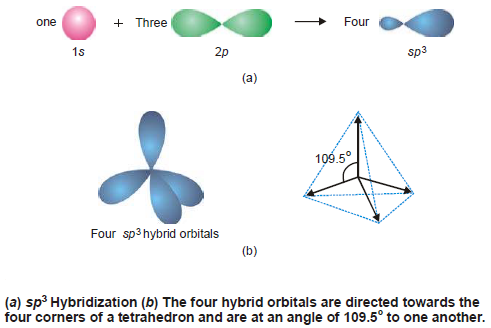
– The four new orbitals formed by mixing an s and three p orbitals of an atom are known as sp3 hybrid orbitals.
– In sp3 hybridization process, Each sp3 hybrid orbital has 25% s-character and 75% p-character.
– Since the mixing of orbitals takes place in space, the four hybrid orbital would also lie in space.
– An arrangement in space that keeps them farthest apart is that of a tetrahedron. Thus each of the four hybrid orbitals is directed towards the four corners of a regular tetrahedron as shown in Fig (b).
– Because of their tetrahedral disposition, this type of hybridization is also called Tetrahedral hybridisation.
– They are of the same shape as that of the previous two types but bigger in size.
– They are disposed in a manner such that the angle between them is 109.5º as shown in the following Figure:
– Examples of sp3 Hybridization: CH4 , SO42-, ClO4– , etc.
Hybridization involving (d) orbitals
– There are several types of hybridization involving d orbitals.
– Since the d orbitals have a relatively complex shape, we will consider here only some of the common types.
– The most important of these are sp3d hybridization, sp3d2 hybridization and sp2d hybridization.
sp3d
– In sp3d hybridization, the orbitals involved are one of s type, three of p type, and one of d type.
– The five new orbitals will be farthest apart by arranging three of them in a plane at an angle of 120º to one another and the other two in a direction perpendicular to the plane.
– The figure obtained by joining the ends assumes the shape of a trigonal bipyramid.
– This type of hybridization is, therefore, called Trigonal bipyramidal hybridization.
sp3d2
– When two (d) type of orbitals take part in hybridisation with one s type and three p type orbitals, six hybrid orbitals called sp3d2 hybrid orbitals are created.
– To be away from one another four of them are dispersed in a plane at an angle of 90º each and the rest two are directed up and below this plane in a direction perpendicular to it.
– On joining their corners, an octahedron results and this type of hybridisation also gets the name Octahedral hybridization
sp2d
– So far we have been considering the hybridisation of orbitals belonging to the same energy level (say 3s, 3p, and 3d orbitals) of an atom. But this may not necessarily be so always.
– In fact, there is very little energy difference between 3d, 4s, and 4p orbitals which may undergo sp2d hybridization.
– The d orbital involved in this type of hybridization has the same planar character as the two p orbitals have and the hybrid orbitals will also be planar, dispersed in such a way so as to be farthest apart i.e., subtending an angle of 90º between them.
– This gives a square planar arrangement for them and the hybridization is, therefore, called Square planar hybridization.
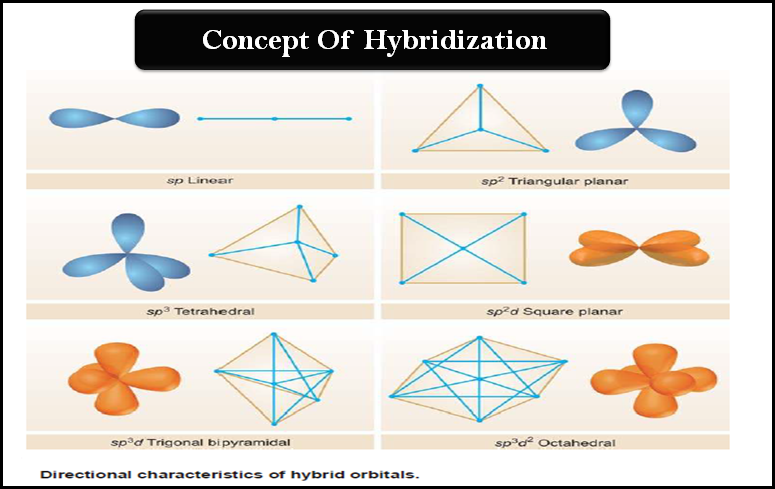
– The directional characters of the types of hybridization discussed above are summarised in the following Figure:
 Read Chemistry
Read Chemistry
The are elaborate and informative 👌